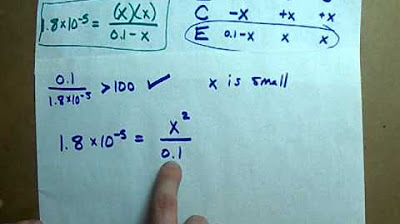How to find pH from molarity and Ka
TLDRThe video script offers a clear and concise guide on calculating the pH of a solution using the molarity and the Ka value. It explains the relationship between Ka, the concentration of H+ ions, and anions, and demonstrates how to rearrange the equation to solve for H+. By applying algebraic manipulation and taking the square root, the video shows how to determine H+ concentration from Ka and molarity. Finally, it illustrates the process of converting H+ concentration to pH using the negative logarithm, providing a step-by-step method to solve such problems efficiently.
Takeaways
- 📝 The script explains the process of calculating pH from molarity and KA value.
- 🔍 The formula for KA is the product of H+ concentration and anion concentration divided by the solution concentration.
- 🧪 The relationship between pH and H+ is given by pH = -log[H+], allowing for the calculation of pH from H+ concentration.
- 📈 The rearranged formula to solve for H+ is √(KA * Molarity), where Molarity is the solution's molarity and KA is the acid dissociation constant.
- 🌟 The example provided in the script uses the values KA = 7.5 × 10^(-3) and Molarity = 0.45.
- 🔢 Solving the rearranged equation yields H+ = 5.8 × 10^(-2) from the given example values.
- 📝 To find the pH, use the formula pH = -log(H+) and substitute the calculated H+ concentration into it.
- 🎯 The calculated pH from the example is 1.2.
- 📊 This method is applicable for both acids and bases, as long as the appropriate dissociation constant (KA or KB) and molarity are known.
- 🔍 Understanding the relationship between pH, pOH, and the ion product of water (Kw) is crucial for these calculations.
- 📚 The script is a practical guide for students or professionals needing to perform acid-base calculations in a laboratory or academic setting.
Q & A
What is the relationship between molarity and pH in the context of the provided transcript?
-In the context of the provided transcript, molarity is used to determine the concentration of H+ ions in a solution, which is then utilized to calculate the pH. The higher the molarity, the higher the concentration of H+ ions, resulting in a lower pH value, indicating a more acidic solution.
What is the formula for the dissociation constant (Ka) mentioned in the transcript?
-The formula for Ka given in the transcript is Ka = [H+] * [A-] / [HA], where [H+] is the concentration of hydrogen ions, [A-] is the concentration of the anion, and [HA] is the concentration of the undissociated acid in the solution.
How can you rearrange the Ka formula to solve for [H+]?
-You can rearrange the Ka formula to solve for [H+] by isolating [H+] on one side of the equation. This can be done by dividing both sides by [HA] and then taking the square root of the resulting expression, which gives [H+] = √(Ka * [HA]).
What is the significance of the square root in the rearranged Ka formula?
-The square root is used in the rearranged Ka formula because the original equation for Ka is a quadratic in terms of [H+]. Taking the square root allows us to solve for the concentration of [H+], which is the concentration of hydrogen ions in the solution.
How is pH related to the concentration of H+ ions?
-pH is related to the concentration of H+ ions by the equation pH = -log[H+]. This means that the pH value is the negative base-10 logarithm of the hydrogen ion concentration, and as [H+] increases (making the solution more acidic), the pH value decreases.
What is the general procedure for calculating pH from molarity and Ka value?
-The general procedure involves first using the Ka value and the molarity of the solution to calculate the concentration of H+ ions using the rearranged Ka formula. Once [H+] is found, it is then used in the pH formula (pH = -log[H+]) to find the pH of the solution.
How does the concentration of the anion ([A-]) affect the calculation of pH?
-The concentration of the anion ([A-]) is a factor in the calculation of Ka, which in turn is used to determine the concentration of H+ ions. As [A-] increases, it indicates a greater degree of dissociation of the acid, leading to a higher [H+] and thus a lower pH value for the solution.
What is the role of the undissociated acid concentration ([HA]) in the Ka formula?
-The concentration of the undissociated acid ([HA]) is part of the Ka formula, representing the amount of the original acid that has not yet dissociated into H+ and A-. It is important in the calculation because it helps to maintain the stoichiometry of the reaction and allows us to calculate the correct concentrations of the species involved.
Why is it important to know the molarity of the solution when calculating pH?
-Knowing the molarity of the solution is important because it provides the initial concentration of the solute, which is necessary for determining the concentrations of H+ ions and the anion after dissociation. This information is crucial for accurately calculating the pH of the solution.
What is the significance of the negative log in the pH calculation?
-The negative log in the pH calculation is used to convert the concentration of H+ ions into a more manageable scale that reflects the acidity or basicity of the solution. The lower the [H+], the higher the pH value, indicating a more basic solution, and vice versa.
How does the value of Ka affect the pH of a solution?
-The value of Ka is a measure of the strength of the acid. A larger Ka value indicates a stronger acid, which will dissociate more completely in solution, leading to a higher concentration of H+ ions and thus a lower pH value. Conversely, a smaller Ka value indicates a weaker acid, resulting in a higher pH value.
Outlines
📚 Calculating pH from Molarity and KA
This paragraph explains the process of calculating the pH of a solution when the molarity and the Ka value are known. It emphasizes the importance of understanding the relationship between Ka (the acid dissociation constant), H+ concentration, and anion concentration. The explanation details the formula for Ka, which is the product of H+ and anion concentrations divided by the solution concentration. It then transitions into the method of finding the H+ concentration from the Ka equation and subsequently using the H+ concentration to calculate the pH, as pH is the negative logarithm of H+. The paragraph also illustrates how to rearrange the equation algebraically to solve for H+, and provides an example calculation leading to a final pH value. The step-by-step approach ensures a clear understanding of the process.
Mindmap
Keywords
💡pH calculation
💡molarity
💡Ka value
💡H+ concentration
💡acid dissociation
💡base stain
💡negative log
💡algebra
💡concentration
💡anion
💡square root
Highlights
The method for calculating the pH of a solution is explained, which is crucial for understanding acid-base chemistry.
The relationship between molarity, the Ka value, and pH is detailed, providing a comprehensive approach to solving related problems.
The formula for Ka is introduced, which is fundamental in determining the concentration of H+ ions in a solution.
The concept of H+ concentration being equivalent to the anion concentration is clarified, simplifying the calculation process.
Algebraic rearrangement of the Ka formula to solve for H+ is described, which is an essential step in finding the pH.
The process of taking the square root of the Ka value and the molarity to find H+ is outlined, providing a clear mathematical procedure.
The method for converting H+ concentration to pH using the negative logarithm is explained, which is key to understanding the acidity or basicity of a solution.
A specific example is worked out, showing how to apply the formulas and calculations to find the pH of a given solution.
The importance of understanding the relationship between pH and H+ is emphasized, as it is vital for various scientific and industrial applications.
The practical application of this method in analyzing and preparing solutions with specific pH levels is highlighted.
The transcript provides a step-by-step guide, which is beneficial for educational purposes and for those new to the subject matter.
The use of the Ka formula in determining the molarity of a solution is discussed, showcasing its versatility in chemical calculations.
The transcript emphasizes the importance of precision in calculations, which is critical in scientific research and experiments.
The process of solving for H+ and subsequently pH is demonstrated, reinforcing the understanding of acid-base equilibrium.
The transcript serves as a valuable resource for anyone needing to understand or teach the principles of acid-base chemistry.
The detailed explanation of the mathematical steps involved in calculating pH from Ka and molarity is provided, enhancing the clarity of the process.
The transcript concludes with a clear example of how to arrive at the pH value, demonstrating the practical application of the discussed concepts.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: