Ka - calculating the pH of a weak acid
TLDRThe video script by Chris Harris from alerts.com explains the process of calculating the pH of a weak acid using the Ka constant. It emphasizes the importance of understanding the dissociation of weak acids and the assumption that the concentrations of H+ and A- are equal at equilibrium. The example calculation demonstrates how to use the Ka expression and the relationship between H+ concentration and pH. The video also highlights the impact of temperature on Ka and the importance of verifying the sensibility of the calculated pH value.
Takeaways
- 📚 The video discusses the calculation of pH for weak acids, highlighting the importance of the Ka constant.
- 🌟 Ka is the acid dissociation constant, a key factor in determining the pH of weak acids due to their partial dissociation in solution.
- 🔄 Weak acids do not fully ionize, leading to an equilibrium that lies to the left, meaning the concentration of H+ does not equal the concentration of the weak acid.
- 📈 The Ka expression is set up as the ratio of products (H+ and the conjugate base) to the reactant (the weak acid), similar to the equilibrium constant Kc.
- 🤔 An assumption is made that the concentrations of H+ and the conjugate base are equal when a weak acid dissociates, simplifying the Ka expression for pH calculation.
- 🧪 The example given involves calculating the pH of a 0.01 moles per DM Cub of ethanoic acid with a Ka value of 1.15 * 10^-5 at a fixed temperature.
- 🌡️ The process of finding the pH involves rearranging the Ka expression to solve for H+ concentration, taking the square root to find H+, and then using the pH formula to calculate the final pH value.
- 📊 The calculated pH of the given example is 3.48, which is consistent with expectations for a weak acid, typically ranging between 2 to 3.
- ⚠️ It's crucial to check the calculated pH value for sensibility; if it's greater than 7, there's likely an error in the calculation.
- 📝 Understanding the assumptions and the reasoning behind them is essential, as they may be examined in an academic setting.
- 🎓 The video provides a clear and concise method for calculating the pH of weak acids, emphasizing the step-by-step approach and the importance of accuracy and verification in the process.
Q & A
What is the main topic of the video?
-The main topic of the video is calculating the pH of a weak acid, with a focus on understanding and applying the Ka expression.
Why can't we assume that the concentration of a weak acid equals the concentration of H+?
-We cannot assume that the concentration of a weak acid equals the concentration of H+ because weak acids dissociate weakly and do not ionize properly, resulting in a significant difference between the concentrations of the acid and the produced H+ ions.
What is the role of the Ka constant in the context of weak acids?
-The Ka constant is the acid dissociation constant used to determine the extent of dissociation of a weak acid. It is the ratio of the concentrations of the products (H+ and the conjugate base) to the concentration of the reactant (the weak acid) at equilibrium.
What is the assumption made in the calculation of pH for weak acids?
-The assumption made is that the concentrations of H+ and the conjugate base (A-) produced from the dissociation of the weak acid (HA) are equal, which allows us to simplify the Ka expression to Ka = [H+]/[HA].
How does temperature affect the Ka value?
-The Ka value can be altered by changes in temperature, as it is a constant that is specific to a given temperature. Different temperatures can result in different Ka values for the same weak acid.
What is the example problem presented in the video?
-The example problem is to calculate the pH of a 0.01 moles per liter solution of ethanoic acid (CH3COOH), with a given Ka value of 1.15 * 10^-5 at a certain temperature.
What is the significance of the equation pH = -log[H+] in the context of acids and bases?
-The equation pH = -log[H+] is fundamental in understanding the acidity or basicity of a solution. It relates the pH, a measure of acidity, to the concentration of H+ ions, which are responsible for acidic properties.
How is the concentration of H+ squared (H+^2) related to the Ka expression?
-In the Ka expression, [H+]^2 represents the concentration of H+ ions squared. This term is derived from the fact that the weak acid (HA) dissociates into H+ and its conjugate base (A-), and at equilibrium, the product of their concentrations equals the Ka constant times the concentration of the weak acid.
What is the step-by-step process for calculating the pH of a weak acid solution?
-The process involves: 1) Establishing the Ka expression, 2) Making the assumption that [H+] = [A-], 3) Rearranging the Ka expression to solve for [H+], 4) Calculating [H+] by taking the square root of [H+]^2, and 5) Using the concentration of H+ in the pH equation to find the pH value.
What is the calculated pH value for the example problem?
-The calculated pH value for the example problem is 3.48, which is a typical pH range for weak acids.
How can you verify if your calculated pH value is correct?
-You can verify the correctness of your calculated pH value by checking if it falls within a sensible range for the type of acid (weak or strong) and ensuring that no mathematical errors were made during the calculation process. If the pH value is greater than 7, it indicates an error in the calculation.
Outlines
📚 Introduction to Calculating pH of Weak Acids
This paragraph introduces the topic of calculating the pH of weak acids, highlighting the importance of the Ka constant. Chris Harris explains that weak acids partially dissociate in solution, which is why the concentration of the weak acid does not equal the concentration of H+ ions. The Ka expression is introduced as a tool to determine the pH of weak acids, and an assumption is made that the concentrations of H+ and the acid's anion are equal. The paragraph sets the stage for an example calculation and emphasizes the significance of the relationship between acids, bases, and their pH values.
🧪 Example Calculation: pH of Ethanoic Acid
In this paragraph, the process of calculating the pH of a specific weak acid, ethanoic acid (CH3COOH), is detailed. The example involves a 0.01 moles per DM cubed solution of ethanoic acid at a given Ka value of 1.15 * 10^-5. The calculation involves using the Ka expression with the assumption that the concentration of H+ squared equals the Ka value times the concentration of the weak acid. After solving for H+ concentration, the square root is taken to find the actual H+ concentration. This value is then used to calculate the pH using the formula pH = -log[H+]. The resulting pH of 3.48 is a sensible value for a weak acid, and the paragraph concludes with advice on checking the reasonableness of the calculated pH value.
Mindmap
Keywords
💡pH
💡weak acid
💡Ka
💡dissociation
💡equilibrium
💡assumption
💡concentration
💡logarithm
💡buffer
💡temperature
💡square root
Highlights
Calculating the pH of a weak acid is the main focus of the video.
Ka is the key constant used in the calculation of weak acids' pH.
Weak acids partially dissociate in solution, leading to an equilibrium that lies to the left.
The Ka expression is introduced as a method to express the acid dissociation constant.
An assumption is made that the concentrations of H+ and A- are equal when a weak acid dissociates.
The relationship between pH and H+ concentration is fundamental in acid-base chemistry.
The example calculation demonstrates the process of determining the pH of a 0.01 moles per DM^3 solution of ethanoic acid.
The value of Ka for ethanoic acid at the given temperature is 1.15 * 10^-5 moles per DM^3.
The calculation involves the rearrangement of the Ka expression to solve for the concentration of H+.
The square root of H+ squared concentration is used to find the actual H+ concentration.
The pH is calculated using the formula pH = -log[H+], where [H+] is the concentration of H+ ions.
The calculated pH for the ethanoic acid solution is 3.48, which is a typical range for weak acids.
It is important to check the sensibility of the calculated pH value to ensure accuracy.
The process of calculating the pH of a weak acid involves several steps, including establishing the Ka expression and making assumptions.
The video provides a clear and detailed explanation of each step in the calculation, making it accessible for learners.
The impact of temperature on the Ka value is acknowledged, indicating that it can change with varying conditions.
The video concludes with a summary of the procedure for calculating the pH of a weak acid, reinforcing the key points.
Transcripts
Browse More Related Video
Ka - Calculating acid concentration and Ka
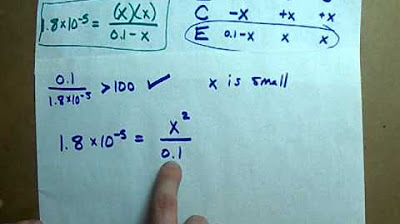
pH of a Weak Acid (0.1 M Acetic Acid) EXAMPLE

Calculating the pH of Weak Acids

Find the Ka of an acid (Given pH) (0.1 M Hypochlorous acid) EXAMPLE

Acid Dissociation Constant, Ka and pKa (A-Level Chemistry)

Calculating the pH of a buffer made from a weak acid and strong base
5.0 / 5 (0 votes)
Thanks for rating: