What's the Difference between Ka and Kb?
TLDRThe video script discusses the concepts of Ka and Kb, which are equilibrium expressions used to describe the behavior of acids and bases in water. Ka is relevant when an acid reacts with water to form H3O+ and its conjugate base, while Kb pertains to the reaction of a base with water to produce OH- and its conjugate acid. The relationship between Ka, Kb, and the ion product of water (KW) is also explained, emphasizing that Ka and Kb are reciprocally related only for conjugate acid-base pairs. The example of HSO4- acting as both an acid and a base illustrates these principles, and the script concludes with the method to calculate Kb from Ka for a given conjugate pair, highlighting the specific conditions under which this relationship holds.
Takeaways
- 📌 The distinction between Ka and Kb lies in their application: Ka is used for acids reacting with water, forming H3O+ and a conjugate base, while Kb is for bases reacting with water, forming OH- and a conjugate acid.
- 🧪 When an acid like HSO4- reacts with water, it donates an H+, resulting in H3O+ and the conjugate base SO4^2-, corresponding to the Ka expression.
- 🧬 The Ka expression for a reaction is given by the concentration of products (like SO4^2-) divided by the concentration of the aqueous reactants (HSO4-).
- 🔄 For a base like HSO4-, the Kb expression represents the reaction with water where it takes an H+, forming H2SO4 and the conjugate acid.
- 🤔 The relationship between Ka and Kb is exemplified by the ion HSO4-, which can act as both an acid (in Ka) and a base (in Kb), depending on its reaction.
- 📈 The product of Ka and Kb for a conjugate acid-base pair equals the ion product of water (KW), but only when dealing with a conjugate pair.
- 🌊 The ion product of water (KW) is the product of the concentrations of H3O+ and OH- in water, and it is only applicable when dealing with conjugate acid-base pairs.
- 📊 The Ka and Kb values are fundamental in understanding the equilibrium of reactions involving acids and bases in aqueous solutions.
- 🔢 To calculate the Kb of a base from its conjugate acid's Ka, use the formula KW = Ka * Kb, and solve for Kb by dividing 10^-14 by the given Ka.
- 🚫 For non-conjugate acid-base pairs, like HSO4- and its supposed conjugates, the relationship between Ka and Kb does not hold as they are not differing by just an H+.
- 🎓 Understanding the concepts of Ka, Kb, and their relationship with KW is crucial for predicting the behavior of acids, bases, and their conjugates in aqueous solutions.
Q & A
What are Ka and Kb, and how do they differ?
-Ka and Kb are both equilibrium expressions used in chemistry. Ka is used when an acid is mixed with water, resulting in the formation of H3O+ (hydronium ion) and the conjugate base of the acid. Kb, on the other hand, is used when a base is mixed with water, leading to the formation of OH− (hydroxide ion) and the conjugate acid of the base.
How does the concept of amphiprotic or amphoteric species relate to Ka and Kb?
-Amphiprotic or amphoteric species can act as both acids and bases in water. For example, HSO4− can donate a proton to water, acting as an acid, or it can accept a proton from water, acting as a base. The Ka expression corresponds to the acid behavior, while the Kb expression corresponds to the base behavior of the amphiprotic species.
What is the chemical reaction for the Ka of HSO4−?
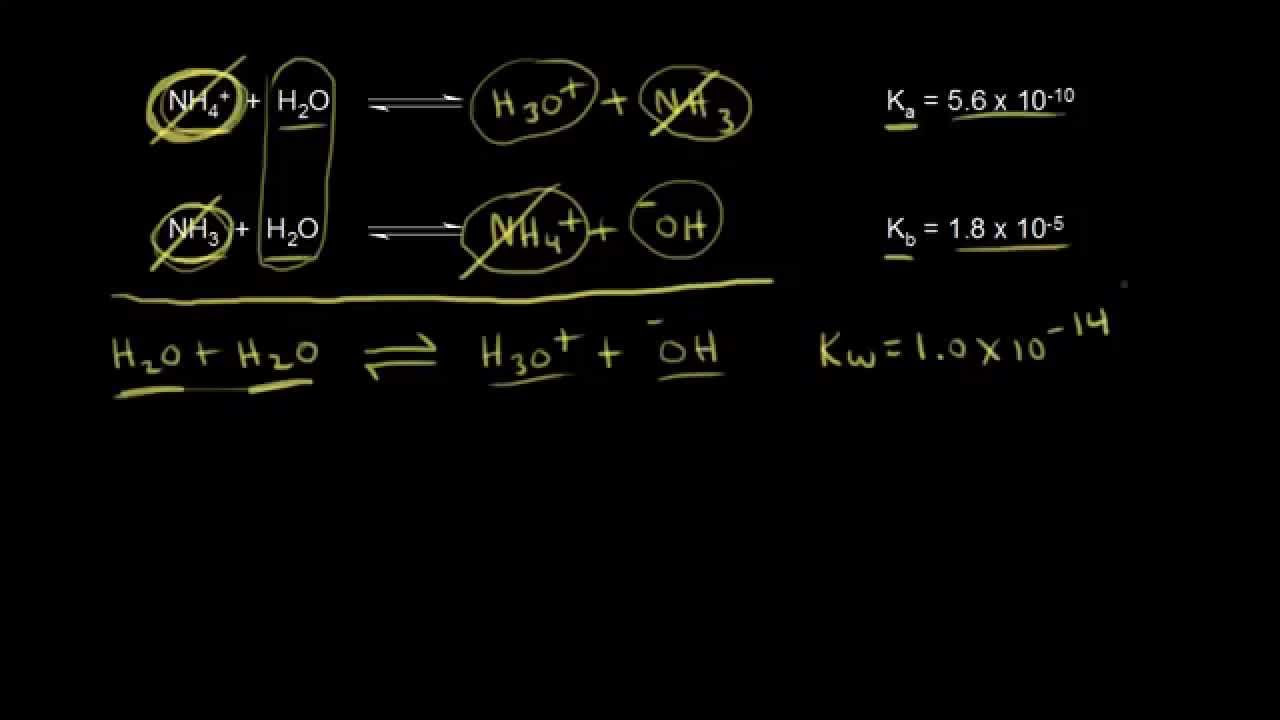
-The chemical reaction for the Ka of HSO4− is HSO4− + H2O ⇌ H3O+ + SO42−. In this reaction, HSO4− acts as an acid, donating a proton to water, resulting in the formation of H3O+ and the conjugate base SO42−.
How is the Ka expression for an acid and its conjugate base related to the Kb expression for the base and its conjugate acid?
-The Ka expression for an acid and its conjugate base is related to the Kb expression for the base and its conjugate acid through the ion product of water (KW). Ka times Kb equals KW, which is a constant at a given temperature. This relationship holds true only when the Ka expression is for the conjugate acid and the Kb expression is for its conjugate base.
What is the significance of the relationship between Ka, Kb, and KW?
-The relationship between Ka, Kb, and KW is significant because it provides a way to calculate the Kb of a conjugate base if the Ka of its conjugate acid is known, and vice versa. This relationship is fundamental in understanding acid-base equilibria in aqueous solutions.
Why does the reaction of HSO4− acting as a base not proceed in theory?
-The reaction of HSO4− acting as a base does not proceed in theory because the product of this reaction, H2SO4, is a strong acid and would not exist in significant concentrations as a proton donor to water. Instead, H2SO4 would readily dissociate into H+ and SO42− ions, with HSO4− being the predominant species in equilibrium with H2SO4.
How can you calculate the Kb of a conjugate base given its Ka?
-You can calculate the Kb of a conjugate base by using the relationship Ka × Kb = KW. If you know the Ka value, you can find the Kb value by dividing 10^−14 (the ion product of water at 25°C) by the Ka value.
What happens when you multiply the Ka and Kb expressions for HF and its conjugate base F−?
-When you multiply the Ka expression for HF (H3O+ × [F−] / [HF]) and the Kb expression for its conjugate base F− ([HF] / [OH−] × [F−]), you get the equilibrium expression for the ion product of water (KW), which simplifies to [H3O+] × [OH−]. This demonstrates the relationship between Ka, Kb, and KW when dealing with conjugate acid-base pairs.
Why are HSO4− and H2SO4 not considered conjugate acids and bases of each other?
-HSO4− and H2SO4 are not considered conjugate acids and bases of each other because they do not differ by a single proton. Conjugate acid-base pairs must have a difference of exactly one hydrogen ion. Instead, HSO4− is the conjugate base of H2SO4, and SO42− is the conjugate base of HSO4−.
What is the role of the hydronium ion (H3O+) in Ka and Kb expressions?
-In Ka and Kb expressions, the hydronium ion (H3O+) represents the product of the acid donating a proton to water when it acts as an acid. In the Ka expression, [H3O+] appears in the numerator as a product, indicating its concentration as a result of the acid-base reaction. In the Kb expression, [H3O+] is not directly involved, as it is the base that is receiving the proton from water, leading to the formation of OH−.
How does the concept of conjugate acid-base pairs apply to the example of HF and F−?
-In the case of HF and F−, HF acts as an acid in water, donating a proton to form H3O+ and F−. Here, F− is the conjugate base of HF. The Ka expression for HF involves [H3O+] and [F−] as products and [HF] as a reactant. The Kb expression for F− involves [HF] and [OH−] as products and [F−] as a reactant. The relationship between Ka and Kb for HF and F− is demonstrated by the fact that their product equals KW, the ion product of water.
Outlines
📚 Understanding Acid-Base Equilibrium: Ka and Kb
This paragraph discusses the concepts of Ka and Kb, which are equilibrium expressions used in chemistry. Ka is relevant when an acid reacts with water to form H3O+, leaving behind its conjugate base. Conversely, Kb applies when a base reacts with water, taking an H+ and forming OH− along with its conjugate acid. The paragraph uses the example of HSO4−, which can act as both an acid and a base in water, to illustrate these reactions and their corresponding equilibrium expressions. It also touches on the relationship between Ka, Kb, and the ion product of water (KW), emphasizing that Ka × Kb = KW only holds true for conjugate acid-base pairs.
🧪 Calculating Ka and Kb for Conjugate Acid-Base Pairs
This paragraph delves into the calculation of Kb from a given Ka, and vice versa, for conjugate acid-base pairs. It explains that the Ka of an acid like HF can be used to calculate the Kb of its conjugate base (F−) by using the constant 10^−14, which is the ion product of water (KW). The paragraph clarifies that this relationship does not apply to non-conjugate pairs, such as HSO4−, which is both the conjugate acid of SO4^2− and the conjugate base of H2SO4. The summary concludes by reiterating that Ka and Kb are related only when they represent conjugate acid-base pairs, differing by a single H+ ion, and highlights the importance of understanding these relationships in chemical equilibrium.
Mindmap
Keywords
💡Equilibrium Expressions
💡Ka
💡Kb
💡Conjugate Acid-Base Pairs
💡Amphoteric
💡Self-ionization of Water
💡Hydronium Ion (H3O+)
💡Sulfuric Acid (H2SO4)
💡Kw
💡Conjugate Acid
💡Conjugate Base
💡Ionization Constant
Highlights
Ka and Kb are equilibrium expressions used in chemistry.
Ka is used when an acid mixes with water, producing H3O+ (hydronium ions) and a conjugate base.
Kb is used when a base mixes with water, taking an H+ from water to form OH- (hydroxide ions) and a conjugate acid.
Amphoteric substances like HSO4- can act as both acids and bases in water.
The chemical reaction of HSO4- acting as an acid is represented by its Ka expression.
The chemical reaction of HSO4- acting as a base is represented by its Kb expression.
Ka and Kb expressions are structured as the ratio of products to reactants.
Kw is the ion product of water, relating the concentrations of H3O+ and OH- in aqueous solutions.
Ka for an acid and its conjugate base, as well as Kb for a base and its conjugate acid, relate to Kw through the equation Ka * Kb = Kw.
HF and its conjugate base F- are used as an example to illustrate the relationship between Ka, Kb, and Kw.
The product of Ka and Kb for a conjugate acid-base pair equals Kw, which is the ion product of water.
The relationship between Ka and Kb does not hold true for non-conjugate acid-base pairs, such as HSO4- and H2SO4.
To calculate the Kb of a conjugate base from its Ka, use the formula 10^-14 divided by the Ka.
The concept of conjugate acids and bases is crucial for understanding the behavior of substances in aqueous solutions.
The Ka and Kb expressions are fundamental in predicting the direction and extent of acid-base reactions.
Understanding the relationship between Ka, Kb, and Kw is essential for various applications in chemistry, such as buffer solutions and pH calculations.
The distinction between Ka and Kb is vital for accurately describing the equilibrium state of acid-base reactions.
The example of HSO4- demonstrates the complexity of acid-base behavior in amphoteric substances.
The discussion of Ka and Kb expressions provides a foundation for further study in chemical equilibrium and reaction dynamics.
Transcripts
Browse More Related Video
Relationship between Ka and Kb | Chemistry | Khan Academy

16.5 pH Calculations for Weak Acids and Bases | General Chemistry

ALEKS - Predicting the Qualitative Acid-Base Properties of Salt

Ka and Kb

pKa and pKb relationship | Acids and bases | Chemistry | Khan Academy
Acid Base Equilibrium Full Topic Video
5.0 / 5 (0 votes)
Thanks for rating: