Chapter 6: Common ion effect | CHM 214 | 057
TLDRThe video script discusses the common ion effect and its impact on solubility. Using mercury chloride in the presence of sodium chloride as an example, it explains how the concentration of mercury ions decreases significantly due to the presence of chloride ions from sodium chloride. The equilibrium concept and the calculation of Ksp are used to demonstrate that the solubility is lower in a solution with a shared ion, highlighting the common ion effect's importance in understanding solubility and equilibria in solutions.
Takeaways
- 🌟 The concept of the common ion effect is introduced, which impacts the solubility of salts in a solution.
- 🔍 Mercury chloride (HgCl2) is used as an example to discuss its solubility in water and the presence of other ions.
- 🧪 At room temperature, the solubility of mercury in a mercury chloride solution is calculated to be 6.7 x 10^-7 molar.
- 📉 In a 0.1 M sodium chloride (NaCl) solution, the concentration of mercury is expected to be lower due to the common ion effect.
- 💧 The presence of chloride ions from NaCl in the solution reduces the solubility of HgCl2, as the chloride ions are already in equilibrium.
- 🔢 The solubility product constant (Ksp) is used to calculate the concentration of mercury, considering the existing chloride concentration.
- 📈 The calculation shows that the mercury concentration in the NaCl solution is 1.2 x 10^-18 M, significantly lower than in pure water.
- 🔄 The common ion effect demonstrates how the presence of a shared ion can decrease the solubility of a salt.
- 📚 Understanding the common ion effect is crucial for predicting the behavior of salts and other compounds in mixed solutions.
- 🌊 The equilibrium in a solution is sensitive to the total concentration of ions, regardless of their source.
- 📉 The presence of other salts in a solution can significantly alter the solubility of the salts present, a phenomenon known as the common ion effect.
Q & A
What is the common ion effect and how does it influence solubility?
-The common ion effect refers to the phenomenon where the presence of an additional ion in a solution, which is common to the dissolution equilibrium of a salt, alters the solubility of that salt. It decreases the solubility of the salt because the common ion from another dissolved substance will reduce the concentration of the salt in solution, thus shifting the equilibrium to favor the undissolved form according to Le Chatelier's principle.
What is the role of sodium chloride in the given example with mercury chloride?
-In the given example, sodium chloride acts as a source of the common ion, chloride, which is also produced when mercury chloride dissolves in water. The presence of chloride ions from sodium chloride suppresses the dissolution of mercury chloride, resulting in a lower concentration of mercury ions in the solution.
How does the presence of sodium chloride in a solution affect the concentration of mercury ions from mercury chloride?
-The presence of sodium chloride, which provides additional chloride ions, reduces the concentration of mercury ions in the solution. This is due to the common ion effect, where the increased concentration of chloride ions shifts the solubility equilibrium of mercury chloride towards the undissolved form, resulting in a lower concentration of mercury ions.
What was the calculated concentration of mercury ions in a mercury chloride solution without sodium chloride?
-The calculated concentration of mercury ions in a mercury chloride solution without sodium chloride was 6.7 times 10 to the power of negative 7 molar.
What is the expected concentration of mercury ions when sodium chloride is also present in the solution?
-When sodium chloride is present in the solution, the expected concentration of mercury ions is significantly lower, at 1.2 times 10 to the power of negative 18 molar, due to the common ion effect.
Why does the solubility of mercury chloride decrease in the presence of sodium chloride?
-The solubility of mercury chloride decreases in the presence of sodium chloride because the additional chloride ions from sodium chloride increase the total chloride ion concentration in the solution. This increased concentration causes the dissolution equilibrium to shift to the left, favoring the undissolved form of mercury chloride and thus reducing the concentration of mercury ions in solution.
How does the common ion effect impact the equilibrium of a solution?
-The common ion effect impacts the equilibrium of a solution by increasing the concentration of a particular ion shared by a dissolved salt. This increased concentration causes the equilibrium to shift in the direction that reduces the production of that common ion, typically by decreasing the solubility of the salt in question.
What is the solubility product constant (Ksp) and how is it related to the common ion effect?
-The solubility product constant (Ksp) is a value that represents the product of the molar concentrations of the ions in a saturated solution at equilibrium. The common ion effect is related to Ksp in that the presence of a common ion from another source in the solution affects the concentrations of the ions from the salt, thus altering the Ksp value and the solubility of the salt.
How does the concentration of mercury ions change when going from a solution with no sodium chloride to one with 0.1 M sodium chloride?
-The concentration of mercury ions decreases significantly, from 6.7 times 10 to the power of negative 7 molar in the absence of sodium chloride to 1.2 times 10 to the power of negative 18 molar in the presence of 0.1 M sodium chloride, due to the common ion effect.
What is the mathematical expression for the solubility product constant (Ksp) for mercury chloride?
-The mathematical expression for the solubility product constant (Ksp) for mercury chloride is Ksp = [Hg^2+][Cl^-], where [Hg^2+] is the molar concentration of mercury ions and [Cl^-] is the molar concentration of chloride ions in the solution.
How does the presence of a common ion from another salt affect the solubility of a given salt?
-The presence of a common ion from another salt reduces the solubility of the given salt. This is because the increased concentration of the common ion shifts the dissolution equilibrium towards the undissolved form of the salt, resulting in a lower concentration of the ions from the given salt in the solution.
Outlines
🌟 Understanding the Common Ion Effect
This paragraph introduces the concept of the common ion effect and its impact on solubility. It uses the example of a mercury chloride solution to explain how the presence of additional chloride ions from a sodium chloride solution affects the equilibrium and solubility of mercury chloride. The key point is that the solubility of mercury chloride decreases significantly when chloride ions are already present in the solution due to the common ion effect, resulting in a much lower concentration of mercury compared to when the salt is dissolved in pure water.
Mindmap
Keywords
💡common ion effect
💡equilibrium
💡Ksp
💡mercury chloride
💡sodium chloride
💡solution
💡solubility
💡concentration
💡molar
💡chemical equilibrium
💡chloride ions
Highlights
Discussion of the common ion effect in solutions.
Use of mercury chloride as an example to explain the common ion effect.
Equilibrium and solubility in the context of mercury chloride solution.
Calculation of mercury concentration in solution at room temperature.
Presence of sodium chloride affecting the concentration of mercury in solution.
Explanation of how chloride ions from sodium chloride impact mercury solubility.
Concept that equilibrium is concerned with the total ion concentration, regardless of source.
Recalculation of mercury concentration in the presence of 0.1 molar sodium chloride solution.
Mathematical demonstration of the decrease in mercury concentration due to the common ion effect.
Significant reduction in mercury solubility in the presence of sodium chloride.
Nine orders of magnitude decrease in mercury concentration as a result of the common ion effect.
Practical implications of the common ion effect on the solubility of salts in solutions with other present ions.
The common ion effect's impact on equilibria in chemical solutions.
Importance of understanding the common ion effect for solubility and equilibrium calculations.
Transcripts
Browse More Related Video

Chapter 6: Precipitate Formation | CHM 214 | 056

Chapter 6: Solubility Product | CHM 214 | 054
Ion Concentration in Solutions From Molarity, Chemistry Practice Problems
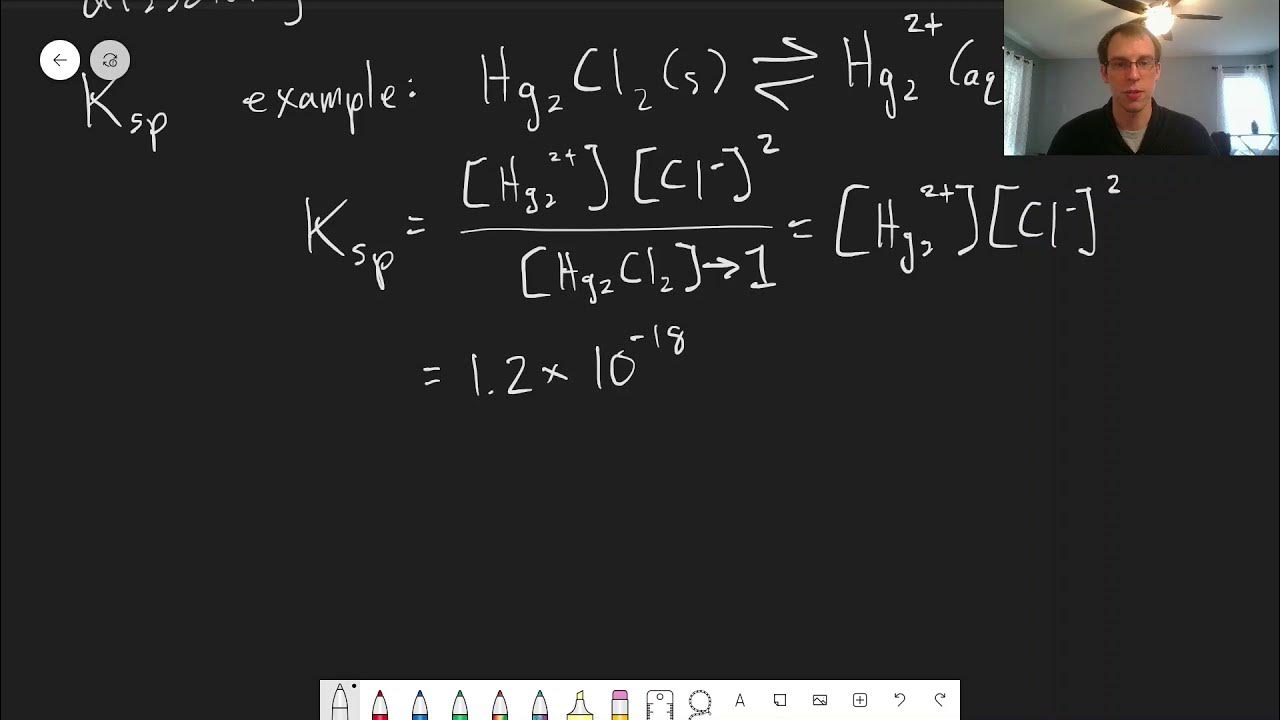
Chapter 6: Solubility Product Example | CHM 214 | 055

Solubility Product Constant (Ksp)

Molecular, complete ionic, and net ionic equations | AP Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: