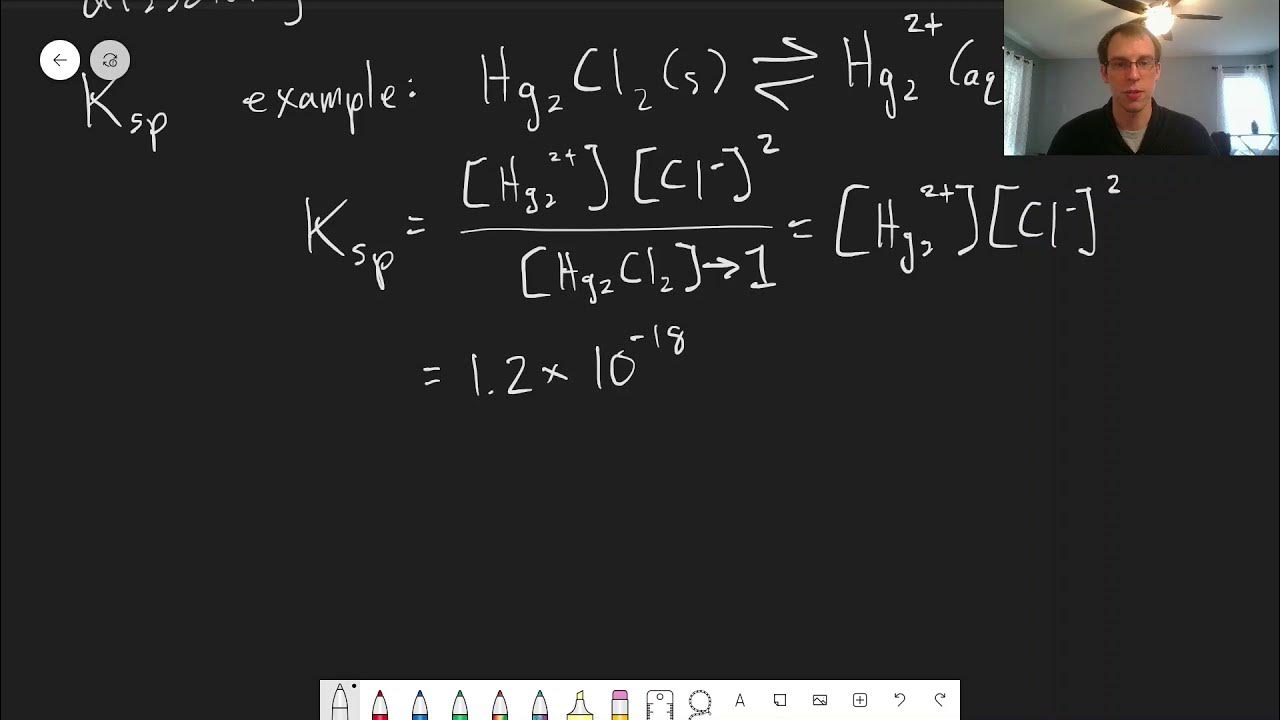Chapter 6: Solubility Product | CHM 214 | 054
TLDRThe transcript discusses the concept of solubility product (Ksp), a specific type of equilibrium constant relevant when a salt dissolves in water. It uses mercury(I) chloride as an example to illustrate how the equilibrium is represented, with the solid on the left and ions on the right. The example shows how the Ksp value, calculated by multiplying the concentrations of the ions raised to their stoichiometric coefficients, can be used to predict whether a salt will dissolve or precipitate. The given Ksp value of 1.2 x 10^-18 for mercury(I) chloride indicates a low solubility, suggesting that the reactants are favored and only minimal concentrations of ions will be present in solution.
Takeaways
- 📚 The discussion revolves around the concept of equilibria, particularly focusing on solubility product (Ksp).
- 🧪 Solubility product is the equilibrium associated with dissolving a salt in water, applicable to aqueous solutions.
- 📈 The equilibrium constant for solubility is given a special name, Ksp, with 'sp' standing for solubility product.
- 📝 In representing the equilibrium, the solid (e.g., mercury(I) chloride) is written on the left side as the reactant.
- 🔄 The equilibrium involves the dissociation of the solid into its constituent ions in solution.
- 🏷️ The mercury(I) ion is a dimer, consisting of two mercury atoms, but has an overall oxidation number of one.
- 🌊 For the solubility product expression, the concentration of mercury(I) ion is raised to the power of one, while the chloride ion concentration is squared due to its stoichiometric coefficient.
- 📊 A pure solid's concentration is considered as one in the solubility product expression, simplifying the equation to just the ion concentrations multiplied with their stoichiometric exponents.
- 🔢 The given example has a solubility product of 1.2 × 10^-18, indicating a low propensity for the salt to dissolve in water.
- 🔄 A small Ksp value suggests that the reactants (solid salt) are favored in the equilibrium, meaning that ion concentrations in solution will be low but not zero.
- 🧠 Understanding Ksp allows us to predict whether a precipitate will form from given ion concentrations in solution, highlighting the importance of studying this type of equilibrium.
Q & A
What is the solubility product and what is its significance in chemistry?
-The solubility product, denoted as Ksp, is an equilibrium constant that describes the dissolution of a salt in water. It is significant because it helps determine the extent to which a salt will dissolve in a solvent, and it can be used to predict whether a precipitate will form from a solution containing specific ions.
How is the solubility product constant (Ksp) written for a salt?
-The Ksp is written by placing the chemical formula of the salt on the left side of the equilibrium arrow, with the solid indicated as such. The product of the concentrations of the constituent ions, each raised to the power of its stoichiometric coefficient in the balanced dissolution equation, is placed on the right side of the arrow.
What does a small Ksp value indicate about a salt's solubility?
-A small Ksp value indicates that a salt is poorly soluble in water, meaning that the equilibrium lies far to the left, favoring the reactants (the solid salt) over the products (the dissolved ions). This results in low concentrations of the ions in solution.
How can the solubility product be used to find the concentrations of ions in solution?
-By knowing the Ksp value of a salt and the concentrations of some of the ions, one can use the solubility product expression to calculate the concentration of the remaining ions. This is done by rearranging the expression and solving for the unknown ion concentration.
What is the role of Ksp in predicting if a precipitate will form in a solution?
-Ksp can be used to predict if a precipitate will form by comparing the ion product (the product of the concentrations of the ions, corrected for their stoichiometry) with the Ksp value. If the ion product exceeds the Ksp, a precipitate will form to reduce the ion concentrations until they are in equilibrium with the Ksp.
How does the presence of a solid salt affect its solubility product expression?
-In the solubility product expression, the presence of a solid salt is indicated by writing the chemical formula of the solid on the left side of the equilibrium arrow. However, the concentration of a pure solid is considered to be constant and is not included in the numerical value of Ksp, which only includes the concentrations of the ions in solution.
What is the relationship between Ksp and the equilibrium constant for a dissolution reaction?
-The Ksp is a specific type of equilibrium constant for the dissolution of a salt into its constituent ions in solution. It is derived from the general equilibrium constant expression by considering the dissolution reaction and the stoichiometry of the ions involved.
How does the solubility of a salt in water differ from its solubility in organic solvents?
-The solubility of a salt can vary greatly between water and organic solvents due to differences in solvent properties such as polarity, dielectric constant, and solvation effects. While water is a polar solvent that generally dissolves ionic compounds well, organic solvents may not provide the same level of solvation for ions, leading to different solubilities.
What is the significance of the stoichiometric coefficients in the solubility product expression?
-The stoichiometric coefficients in the solubility product expression are crucial because they determine the powers to which the ion concentrations are raised. This reflects the ratio of the moles of each ion produced when the salt dissolves, and it ensures that the product of the ion concentrations accurately represents the equilibrium between the solid salt and its ions in solution.
What is an example of a salt with a small Ksp value and what can be inferred from its solubility in water?
-Mercury(I) chloride (Hg2Cl2) has a Ksp value of 1.2 × 10^-18, which is very small. This indicates that it is largely insoluble in water, and only very low concentrations of Hg2+ and Cl- ions will be present in a saturated solution of this salt.
How does the concept of solubility product apply to sparingly soluble salts?
-Solubility product constants are particularly applicable to sparingly soluble salts, which are salts that have low solubility in water. The Ksp values for these salts are small, indicating that their dissolution is limited and that the equilibrium lies far to the left, favoring the solid form over the dissolved ions.
Outlines
🔬 Introduction to Solubility Product
This segment introduces the concept of solubility product (Ksp), a specific equilibrium related to the dissolving of salts in water, though it can also apply to other solvents. Ksp is described through the example of mercury(I) chloride dissolving in water, where the solid salt dissociates into its constituent ions in an aqueous solution. The formula for calculating Ksp is explained, emphasizing that it only includes the concentrations of the ions in solution, raised to the power of their stoichiometric coefficients. A specific Ksp value (1.2 x 10^-18) illustrates that, for this salt, the equilibrium heavily favors the solid form due to the very low solubility product, indicating very low concentrations of dissolved ions. This discussion underscores the significance of Ksp in predicting whether a salt will remain dissolved or form a precipitate in solution.
Mindmap
Keywords
💡equilibria
💡solubility product (Ksp)
💡aqueous solutions
💡salts
💡ions
💡stoichiometric coefficients
💡concentrations
💡precipitate
💡reactants
💡products
💡equilibrium constant
Highlights
Discussion of equilibria basics and specific examples in the class.
Focus on the solubility product, an equilibrium associated with dissolving a salt in water.
The equilibrium constant for solubility product is given a special name, Ksp.
Solubility product can be applied to aqueous solutions and organic solvents.
The solid reactant in solubility product equilibria is written on the left side.
Example given of mercury(I) chloride dissociating into its constituent ions in solution.
Explanation of how to write the Ksp expression for a solubility product reaction.
Mention of stoichiometric coefficients and their role in the Ksp expression.
Pure solids are represented as '1' in the solubility product expression.
Solubility product is calculated as the concentration of ions raised to their stoichiometric coefficients.
The solubility product value for mercury(I) chloride is given as 1.2 x 10^-18.
A small Ksp value indicates that the salt will primarily stay as a solid.
The equilibrium constant being very small means the reactants are favored.
The presence of non-zero concentrations of ions in solution is discussed.
The concept of determining if ions in solution will form a precipitate or remain in solution is introduced.
The importance and well-studied nature of solubility product equilibrium is highlighted.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: