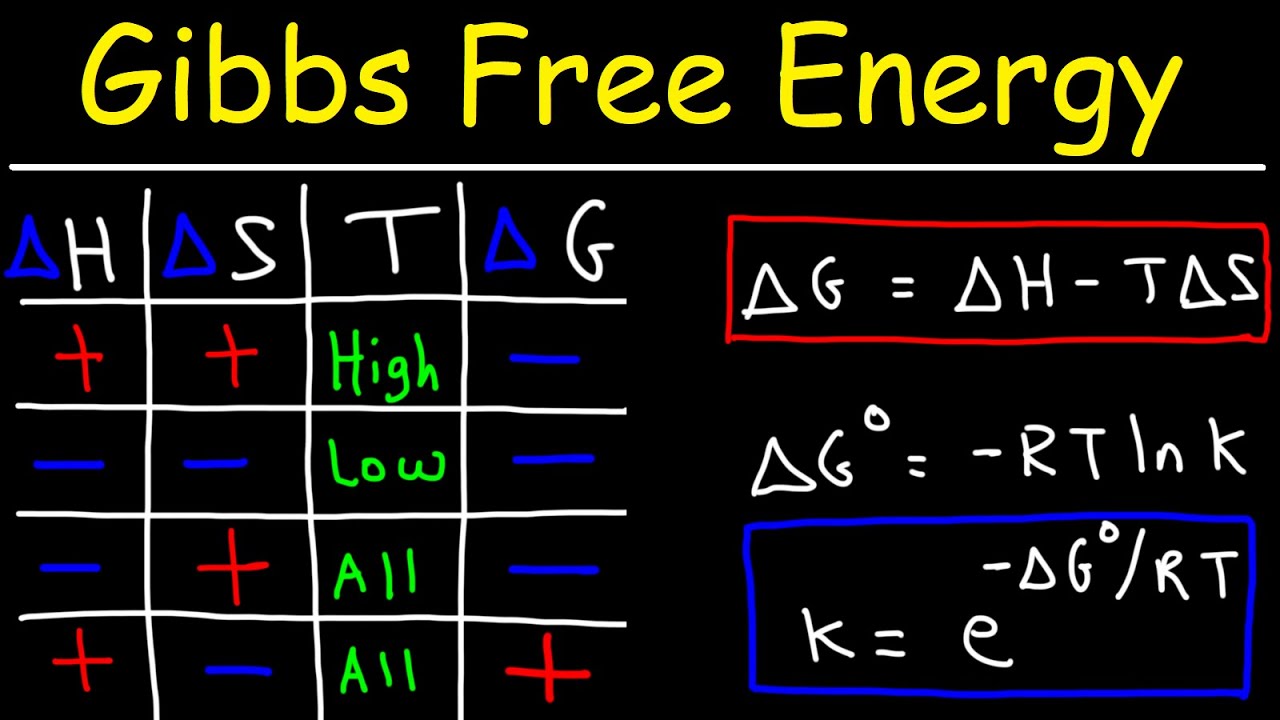
Exergonic vs endergonic reaction diagrams
TLDRThe video script discusses exergonic and endergonic reactions, explaining their representation on diagrams with Gibbs free energy (ΔG) on one axis and reaction progress on the other. It clarifies that endergonic reactions have a positive ΔG, absorbing energy as they proceed from simpler to more complex molecules, while exergonic reactions have a negative ΔG, releasing energy as they break down complex molecules into simpler ones. The script also touches on the role of enzymes in lowering the activation energy (ΔG of activation) for these reactions, facilitating their progress.
Takeaways
- 📈 Exergonic and endergonic reactions are represented on diagrams with Delta G on one axis and reaction progress on the other.
- 📉 In an endergonic reaction, Delta G is lower in the reactants than in the products, indicating energy is absorbed from the surroundings.
- 🔄 In an exergonic reaction, Delta G is higher in the reactants than in the products, meaning energy is released to the surroundings.
- 🅿️ The total change in Delta G represents the overall energy change during the reaction.
- 🌐 Gibbs free energy is used to describe the energy exchange in these reactions.
- 🚀 Energy of activation is the Delta G between the reactants and the highest point (peak) of the reaction, representing the energy needed to initiate the reaction.
- 🌟 Enzymes and catalysts can lower the energy of activation, thus facilitating the reaction.
- 📈 Dehydration reactions are anabolic and endergonic, involving the build-up from simple to complex molecules, requiring energy input.
- 📉 Hydrolysis reactions are catabolic and exergonic, involving the breakdown from complex to simple molecules, releasing energy.
- 🔄 The nature of a reaction (endothermic or exothermic) can be deduced from the shape and values on the reaction diagram.
- 📚 Understanding these energy diagrams is crucial for grasping the thermodynamics of biochemical reactions.
Q & A
What is the primary difference between exergonic and endergonic reactions in terms of energy exchange with the surroundings?
-Exergonic reactions release energy to the surroundings, while endergonic reactions absorb energy from the surroundings. In exergonic reactions, the reactants have higher Gibbs free energy than the products, whereas in endergonic reactions, the products have higher Gibbs free energy than the reactants.
How is the change in Gibbs free energy (ΔG) represented graphically in the energy diagrams for exergonic and endergonic reactions?
-In the energy diagrams, the y-axis represents ΔG, and the x-axis represents reaction progress or time. For exergonic reactions, the graph shows a downward slope, indicating a negative ΔG as the reaction proceeds from reactants to products. Conversely, endergonic reactions are represented by an upward slope, indicating a positive ΔG.
What is the significance of the activation energy in the context of exergonic and endergonic reactions?
-Activation energy is the minimum energy required to initiate a reaction. It represents the energy barrier that must be overcome for the reaction to proceed. In exergonic reactions, the activation energy is typically lower because the reaction naturally releases energy, making it easier to overcome the barrier. In endergonic reactions, the activation energy is higher since energy must be absorbed to proceed.
How do enzymes affect the activation energy of chemical reactions?
-Enzymes act as catalysts and can lower the activation energy required for a reaction to occur. By providing an alternative reaction pathway with a lower activation energy, enzymes increase the rate of both exergonic and endergonic reactions without being consumed in the process.
What is the relationship between hydrolysis and dehydration reactions in terms of energy flow?
-Hydration reactions are typically endergonic, as they require energy input to break bonds and add water to a molecule. On the other hand, dehydration reactions are usually exergonic, as they release energy by removing water and forming simpler molecules.
How do anabolic and catabolic processes relate to endergonic and exergonic reactions?
-Anabolic processes involve the formation of larger, more complex molecules from simpler ones, which is an endergonic reaction as it requires energy input. Catabolic processes involve the breakdown of complex molecules into simpler ones, which is an exergonic reaction because it releases energy.
What is the significance of the entropy change in exergonic and endergonic reactions?
-Entropy is a measure of the disorder or randomness of a system. In exergonic reactions, entropy typically increases as the system becomes more disordered, while in endergonic reactions, entropy usually decreases as the system becomes more ordered.
How does the spontaneity of a reaction relate to its ΔG value?
-A negative ΔG value indicates a spontaneous reaction, as it proceeds naturally without the need for external energy input. A positive ΔG value indicates a non-spontaneous reaction, which requires energy input to proceed. A ΔG of zero indicates that the system is at equilibrium, with no net change in energy.
What is the role of Gibbs free energy in determining the spontaneity of a reaction?
-Gibbs free energy (ΔG) is a thermodynamic potential that combines enthalpy (heat content) and entropy (disorder) to determine whether a process will occur spontaneously. A negative ΔG indicates a spontaneous process, a positive ΔG indicates a non-spontaneous process, and a ΔG of zero indicates equilibrium.
How can the energy diagrams of exergonic and endergonic reactions be used to predict the direction of a reaction?
-By observing the slope of the energy diagram, one can predict the direction in which a reaction will proceed to reach equilibrium. A downward slope indicates an exergonic reaction that releases energy, while an upward slope indicates an endergonic reaction that absorbs energy. The reaction will proceed in the direction that reduces the system's Gibbs free energy.
What is the difference between exothermic and endothermic reactions in relation to exergonic and endergonic reactions?
-Exothermic reactions are a type of exergonic reactions where energy is released in the form of heat. Endothermic reactions are not necessarily endergonic; they absorb energy but do not always result in an increase in Gibbs free energy. However, all endergonic reactions are endothermic to some extent since they require energy input.
Outlines
📊 Understanding Exergonic and Endergonic Reaction Diagrams
This paragraph introduces the concept of exergonic and endergonic reactions, represented graphically with delta G on one axis and reaction progress on the other. It explains how the position of the reactants and products on the delta G axis indicates whether a reaction is endergonic (positive delta G) or exergonic (negative delta G). The paragraph also touches on the role of enzymes and catalysts in lowering the activation energy (delta G of activation) required to initiate a reaction.
Mindmap
Keywords
💡Exergonic reaction
💡Endergonic reaction
💡Delta G
💡Reaction progress
💡Energy of activation
💡Catabolic reaction
💡Anabolic reaction
💡Hydrolysis
💡Dehydration
💡Metabolism
💡Enzymes
Highlights
The discussion revolves around exergonic and endergonic reaction diagrams, providing insights into their graphical representation and underlying principles.
The axis representation in both diagrams is identical, with delta G on one axis and reaction progress or time on the other.
A reaction where delta G is lower in reactants than in products is identified as an endergonic reaction, indicating energy absorption from the surroundings.
The total change in delta G is the key indicator of whether a reaction is endergonic or exergonic, with positive values for endergonic and negative for exergonic.
Exergonic reactions are characterized by the release of energy to the universe, as delta G is given off from reactants to products.
The energy of activation is defined as the delta G between the reactants and the highest point (peak) of the reaction curve.
Enzymes and catalysts can lower the energy of activation, thus facilitating the progress of reactions.
Dehydration reactions are anabolic and endergonic, involving the build-up from simple to complex molecules and requiring energy input.
Hydrolysis reactions are catabolic and exergonic, breaking down complex molecules into simpler ones and releasing energy in the process.
The energy diagrams provide a visual and intuitive understanding of the energy dynamics in biochemical reactions.
Understanding the differences in delta G is crucial for predicting the spontaneity and feasibility of biochemical reactions.
The lecture emphasizes the importance of recognizing the type of reaction (anabolic or catabolic) by analyzing the energy diagrams.
The relationship between the energy of activation and the effectiveness of enzymes and catalysts is highlighted, showcasing their role in metabolic processes.
The practical applications of understanding exergonic and endergonic reactions extend to fields such as biochemistry, molecular biology, and metabolic engineering.
The session concludes with a comprehensive overview of energy diagrams, reinforcing their significance in the study of biochemical reactions.
Transcripts
Browse More Related Video
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

Endergonic and Exergonic Reactions; Feedback Inhibition

ATP and Coupled Reactions

Gibbs free energy and spontaneous reactions | Biology | Khan Academy

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry
[H2 Chemistry] 2021 Topic 5 Energetics 3
5.0 / 5 (0 votes)
Thanks for rating: