13.04 Isotopic Abundance in Mass Spectrometry
TLDRThe video script discusses the impact of isotopes on mass spectrometry in organic chemistry, with a focus on chlorine and bromine due to their significant isotopic abundance. It explains that while most elements have a single dominant isotope, the presence of multiple isotopes in chlorine and bromine leads to distinct mass spectrum peaks. For instance, bromine has isotopes bromine-79 and bromine-81 in a 1:1 ratio, resulting in two molecular ion peaks in the mass spectrum for bromobenzene at 156 and 158. Similarly, chlorobenzene shows two peaks due to the presence of chlorine-35 and chlorine-37 in a 3:1 ratio. The script also touches upon the statistical patterns observed in molecules with multiple chlorine atoms, highlighting the complexity of isotopic effects on mass spectrometry analysis.
Takeaways
- 🌟 Most elements in organic chemistry have a single dominant isotope, simplifying the interpretation of mass spectra.
- ⚠️ Chlorine and bromine are exceptions with significant natural abundance of multiple isotopes, affecting mass spectra interpretation.
- 📊 Chlorine has isotopes Chlorine-35 and Chlorine-37 in a natural ratio of about 3:1.
- 📈 Bromine has isotopes Bromine-79 and Bromine-81 with a nearly 1:1 natural abundance ratio.
- 🧬 The different masses of these isotopes result in distinct peaks on a mass spectrum, providing insight into the number of halogen atoms in a molecule.
- 💡 For bromobenzene, two molecular ion peaks are observed due to the presence of Bromine-79 and Bromine-81 isotopes.
- 🤔 The lower mass peak corresponds to molecules with the Bromine-79 isotope, and the higher mass peak corresponds to Bromine-81.
- 🔍 Statistical effects are observed in molecules with multiple bromine atoms, but the peak pattern near the molecular ion still reflects the isotope probabilities.
- 🌀 For chlorobenzene, the mass spectrum shows two apparent molecular ion peaks due to the abundance of Chlorine-35 and Chlorine-37.
- 📐 The height of the peak at lower mass due to Chlorine-35 is about three times that of the peak at higher mass due to Chlorine-37.
- 🧮 In molecules with multiple chlorines, a statistical pattern is observed, but the interpretation is more complex due to the varying probabilities of observing each isotope.
Q & A
Why is it generally not a concern to consider different isotopes when studying most elements in organic chemistry?
-For most elements in organic chemistry, a single isotope is dominant, which means that different isotopes do not usually appear in different molecules to affect the mass spectrum significantly.
Which two elements in organic chemistry are exceptions to the rule of single isotope dominance?
-Chlorine and bromine are the two exceptions, as they both have multiple isotopes present in significant abundance.
What are the naturally occurring isotopes of chlorine and their approximate ratio?
-Chlorine has two naturally occurring isotopes, chlorine-35 and chlorine-37, with an approximate ratio of three to one.
What are the naturally occurring isotopes of bromine and their approximate ratio?
-Bromine has two naturally occurring isotopes, bromine-79 and bromine-81, with an approximate ratio of one to one.
How do the different masses of isotopes for chlorine and bromine affect the mass spectrum of a molecule?
-The different masses of isotopes for chlorine and bromine result in different peaks on a mass spectrum, which can provide insight into the number of chlorine or bromine atoms within a molecule.
What is observed in the mass spectrum of bromobenzene regarding the molecular ion peak?
-In the mass spectrum of bromobenzene, two apparent molecular ion peaks are observed: one at M/Z = 156 and another at M/Z = 158, which are due to the presence of bromine-79 and bromine-81 isotopes.
What is the significance of the two molecular ion peaks in the mass spectrum of bromobenzene?
-The two peaks indicate a 50/50 ratio of bromine-79 and bromine-81 isotopes in the sample, with the lower mass peak corresponding to molecules containing bromine-79 and the higher mass peak to molecules containing bromine-81.
How do statistical effects come into play for molecules containing multiple bromine atoms?
-For molecules with multiple bromine atoms, the peak pattern near the molecular ion still reflects a 50% probability of seeing bromine-79 and a 50% probability of seeing bromine-81 at each bromine site.
What is the theoretical mass spectrum of chlorobenzene like?
-The theoretical mass spectrum of chlorobenzene displays two apparent molecular ion peaks—one at 112 and another at 114 mass units higher—due to the presence of chlorine-35 and chlorine-37 isotopes.
How does the abundance of chlorine-35 and chlorine-37 isotopes affect the mass spectrum of molecules containing chlorine?
-The mass spectrum shows a statistical pattern with the peak at lower mass (due to molecules containing chlorine-35) being about three times as high as the peak at higher mass (due to molecules containing chlorine-37), reflecting the 75% probability of observing chlorine-35 and 25% for chlorine-37.
Why are the peak patterns near the molecular ion in the mass spectrum of molecules with multiple chlorines more complicated?
-The peak patterns are more complicated because the probability of observing chlorine-35 is 75% and chlorine-37 is 25%, leading to a more complex statistical distribution in the observed masses.
How do the isotopic peak patterns in mass spectra provide additional information about the sample?
-The isotopic peak patterns allow for the determination of the number of chlorine or bromine atoms within a molecule by analyzing the relative intensities and positions of the peaks.
Outlines
🌟 Isotopes in Organic Chemistry
This paragraph discusses the dominance of a single isotope in most elements of organic chemistry and the exceptions, specifically chlorine and bromine, which have multiple isotopes in significant abundance. It explains how these isotopes with different masses give different peaks on a mass spectrum, providing insight into the number of chlorine or bromine atoms within a molecule. The example of bromobenzene is used to illustrate this, with two molecular ion peaks at M/Z ratios of 156 and 158, attributed to the nearly equal abundance of bromine-79 and bromine-81 isotopes. The paragraph also touches on statistical effects in molecules containing multiple bromine atoms and compares the pattern observed in chlorobenzene due to the abundance of chlorine-35 and chlorine-37 isotopes.
Mindmap
Keywords
💡Isotope
💡Mass Spectrum
💡Organic Chemistry
💡Chlorine-35 and Chlorine-37
💡Bromine-79 and Bromine-81
💡Molecular Ion Peak
💡Isotopic Abundance
💡Statistical Effects
💡Fragmentation
💡Coupling Statistics
💡Probabilities
Highlights
Most elements in organic chemistry have a single dominant isotope, simplifying mass spectrometry analysis.
Chlorine and bromine are exceptions with multiple significant isotopes naturally occurring.
Chlorine-35 and chlorine-37 exist in a ratio of approximately three to one.
Bromine-79 and bromine-81 exist in a near one to one ratio.
Different masses of isotopes result in distinct peaks on a mass spectrum.
Peak patterns near the molecular ion can indicate the number of chlorine or bromine atoms in a molecule.
Bromobenzene's mass spectrum shows two molecular ion peaks due to isotopic abundance effects.
The lower mass peak corresponds to molecules with bromine-79, and the higher to bromine-81.
Statistical effects influence peak patterns in molecules with multiple bromine atoms.
There is a 50% probability of encountering each bromine isotope in the molecular ion peak pattern.
Coupling statistics from NMR context are reminiscent but not related to isotopic peak patterns.
Chlorobenzene's spectrum shows two apparent molecular ion peaks due to the abundance of chlorine isotopes.
The peak for molecules with chlorine-35 is about three times higher than that with chlorine-37.
The probability of observing chlorine-35 is 75%, and for chlorine-37 is 25%, complicating the statistics.
The mass spectrum reflects the isotopic composition of the sample, providing insight into molecular structure.
Isotopic peak patterns offer a unique tool for identifying and quantifying elements within organic compounds.
Understanding isotopic effects is crucial for accurate mass spectrometry analysis in organic chemistry.
The transcript provides a detailed explanation of isotopic effects on mass spectra in organic chemistry.
Transcripts
Browse More Related Video

14.5 Isotope Effects in Mass Spectrometry | Organic Chemistry

15.6e Structural Determination From All Spectra Example 5 | Organic Chemistry

Basics of mass Spectroscopy. How to estimate the formula from molar mass. (M+ and M+1, M+2 )
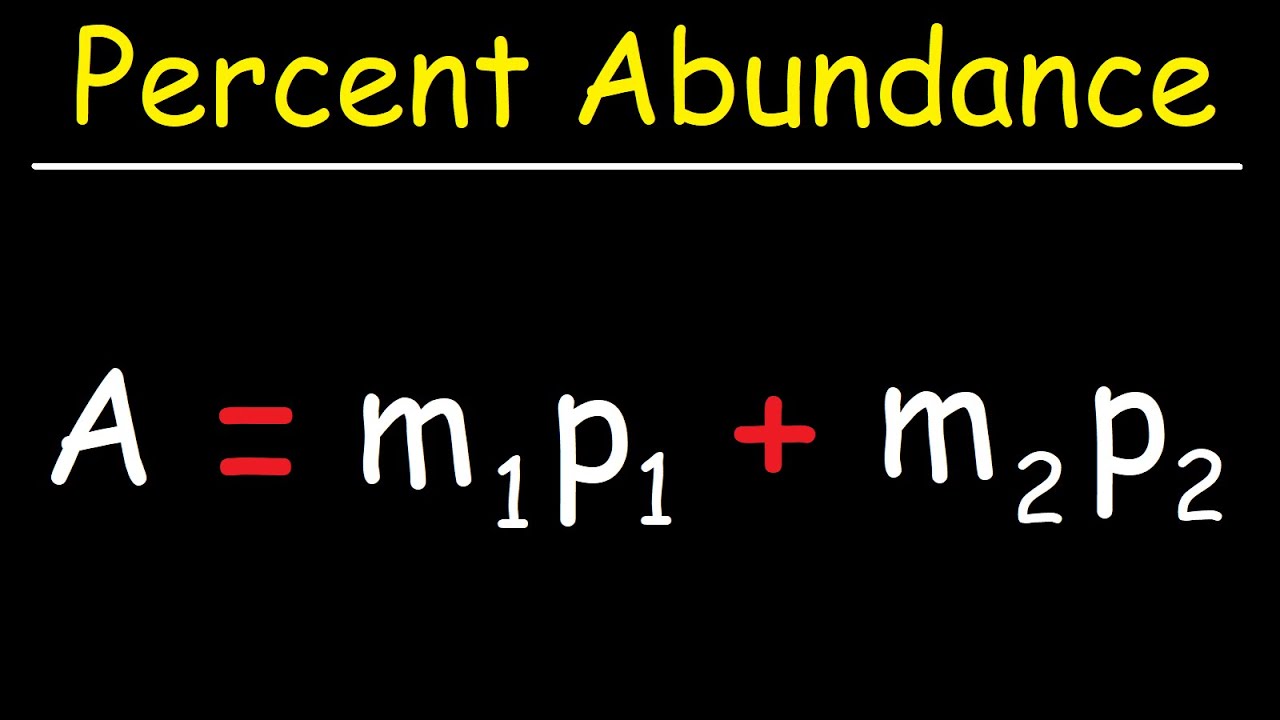
How To Find The Percent Abundance of Each Isotope - Chemistry

15.6d Structural Determination From All Spectra Example 4 | Organic Chemistry

Atomic Mass: How to Calculate Isotope Abundance
5.0 / 5 (0 votes)
Thanks for rating: