How To Calculate The Average Atomic Mass
TLDRThe video script explains the method for calculating the average atomic mass of an element using its isotopes' masses and their respective abundance percentages. It demonstrates this with examples of chlorine, magnesium, and iron isotopes, showing how to apply the formula and deduce the most abundant isotope based on its mass proximity to the average atomic mass. The explanation is clear, engaging, and informative, making it easy for viewers to understand and apply the concept.
Takeaways
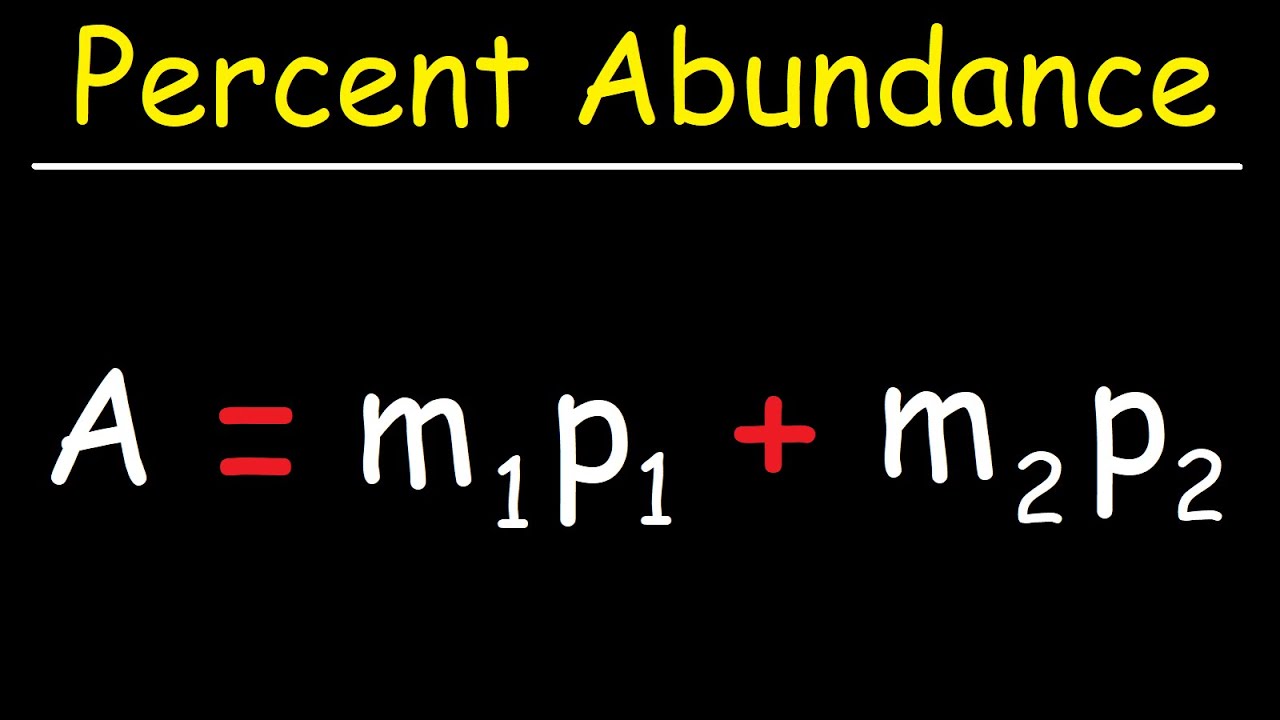
- 📊 The average atomic mass of an element can be calculated using a simple formula involving the mass of its isotopes and their respective percentage abundances.
- 💡 Isotopes' percentage abundances need to be converted to decimal form by dividing by 100 or moving the decimal point two places to the left.
- 🌟 For chlorine, the average atomic mass is calculated by multiplying the atomic mass of each isotope (34.96 amu for Cl-35 and 36.966 amu for Cl-37) by their respective percentage abundances (0.7576 for Cl-35 and 0.2424 for Cl-37) and summing the results.
- 🏆 The resulting average atomic mass for chlorine in the example is 35.453 amu.
- 🔍 The most abundant isotope of an element can often be deduced by comparing its atomic mass to the average atomic mass; the closest usually being the most abundant.
- 🧪 Magnesium's most abundant isotope is Mg-24, as its atomic mass (24.305 amu) is closest to the average atomic mass of magnesium (24.305 amu).
- 🌐 Iron has four stable isotopes, and their average atomic mass is calculated similarly to chlorine, by multiplying each isotope's mass by its percentage abundance and summing these values.
- 🤔 The average atomic mass of iron is found to be 55.845 amu, which is very close to the mass of the most abundant isotope, Fe-56.
- 📝 When calculating average atomic masses, precision is crucial as even small mistakes can significantly alter the result.
- 🎓 Understanding the relationship between average atomic mass and the abundance of isotopes is fundamental for grasping the composition of elements.
- 👋 The video concludes by reinforcing the method for calculating the average atomic mass of an element, providing viewers with a clear and practical approach.
Q & A
What are the two isotopes of chlorine mentioned in the example problem?
-The two isotopes of chlorine mentioned are chlorine-35 and chlorine-37.
How is the average atomic mass of chlorine calculated using its isotopes?
-The average atomic mass of chlorine is calculated by multiplying the mass of each isotope by its percentage abundance in decimal form and then summing these products.
What is the atomic mass unit (amu) of chlorine-35?
-The atomic mass unit of chlorine-35 is 34.96 amu.
How do you convert the percentage abundance of an isotope into its decimal form?
-To convert the percentage abundance into its decimal form, divide the percentage by 100 or move the decimal point two places to the left.
What is the average atomic mass of chlorine as calculated in the example?
-The average atomic mass of chlorine is 35.453 amu.
Which isotope of magnesium is most abundant based on its average atomic mass?
-Magnesium-24 is the most abundant isotope since its mass is closest to the average atomic mass of magnesium, which is 24.305.
How many stable isotopes does iron have according to the problem?
-Iron has four stable isotopes as mentioned in the problem.
What is the formula used to calculate the average atomic mass of an element with multiple isotopes?
-The formula is the sum of the products of the mass of each isotope and its percentage abundance in decimal form.
What is the average atomic mass of iron as calculated in the example?
-The average atomic mass of iron is 55.845 amu.
Which iron isotope is most abundant based on the average atomic mass?
-Fe-56 is the most abundant isotope since it has the highest percentage abundance and its mass is closest to the average atomic mass of iron.
Why is the most abundant isotope typically the one closest to the average atomic mass?
-The most abundant isotope is typically the one closest to the average atomic mass because the average atomic mass is calculated based on the relative abundances and atomic masses of all isotopes of the element.
How can the method of calculating average atomic mass be applied to other elements?
-The same method of calculating the average atomic mass by using the formula that involves multiplying each isotope's mass by its percentage abundance and summing these products can be applied to any element with multiple isotopes.
Outlines
📊 Calculating Chlorine's Average Atomic Mass
This paragraph explains the process of calculating the average atomic mass of chlorine using the given data on its isotopes, chlorine 35 and chlorine 37. The method involves multiplying the atomic mass of each isotope by its percentage abundance (in decimal form) and summing the results. The example provided uses the mass of 34.96 amu for chlorine 35 with a 75.76% abundance and the mass of 36.966 amu for chlorine 37 with a 24.24% abundance. The calculation yields an average atomic mass of 35.453 for chlorine.
🔍 Determining the Most Abundant Isotope of Magnesium
The second paragraph discusses how to identify the most abundant isotope of magnesium based on its average atomic mass of 24.305. The three stable isotopes of magnesium, magnesium 24, magnesium 25, and magnesium 26, are considered, and the isotope with the mass closest to the average (magnesium 24) is determined to be the most abundant. This section also introduces a more complex example involving four stable isotopes of iron, providing a brief overview of the calculation process for determining iron's average atomic mass.
Mindmap
Keywords
💡Isotopes
💡Atomic Mass
💡Percent Abundance
💡Average Atomic Mass Formula
💡Magnesium
💡Iron
💡Element Abundance
💡Atomic Mass Units (amu)
💡Chemical Elements
💡Scientific Calculation
Highlights
Chlorine has two isotopes, chlorine 35 and chlorine 37.
The percent abundance and atomic mass of each chlorine isotope are provided.
A formula is used to calculate the average atomic mass of chlorine based on isotope mass and abundance.
The mass of the first chlorine isotope (chlorine 35) is 34.96 amu.
The percentage abundance of chlorine 35 is 75.76%, which is converted to 0.7575 as a decimal.
The mass of the second chlorine isotope (chlorine 37) is 36.966 amu.
The percentage abundance of chlorine 37 is 24.24%, which is converted to 0.2424 as a decimal.
The average atomic mass of chlorine is calculated to be 35.453.
Magnesium has three stable isotopes: magnesium 24, magnesium 25, and magnesium 26.
The average atomic mass of magnesium is given as 24.305.
The most abundant isotope of magnesium is the one closest to the average atomic mass, which is Mg24.
Iron metal has four stable isotopes, and the average atomic mass is calculated using a similar formula.
The average atomic mass of iron is calculated to be 55.845.
The most abundant isotope of iron is Fe 56, which has a percentage abundance of 91.754%.
The average atomic mass of an element can be determined by calculating the weighted average of its isotopes' masses and abundances.
The isotope closest to the average atomic mass is usually the most abundant.
The method demonstrated is a fundamental concept in understanding the atomic composition of elements.
Transcripts
Browse More Related Video

Isotopes, Percent Abundance, Atomic Mass | How to Pass Chemistry

Atomic Mass: How to Calculate Isotope Abundance
How To Find The Percent Abundance of Each Isotope - Chemistry

What's the Difference between Mass Number and Atomic Mass?

Atomic Mass: Introduction

Atomic Number, Mass Number, and Net Electric Charge
5.0 / 5 (0 votes)
Thanks for rating: