Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (89 of 92) Which Penetrates Easier?
TLDRThe video script explores the transmission coefficient and the probability of particles, specifically electrons and protons, passing through a barrier. It compares the two scenarios where both particles have the same energy but different masses, resulting in different probabilities of passing the barrier. The script explains that due to its larger mass, a proton has a smaller chance of getting through the barrier compared to an electron. The mathematical calculations provided illustrate this concept, showing that while 15.7% of electrons might pass through, virtually no protons would, highlighting the impact of particle mass on the likelihood of barrier penetration.
Takeaways
- 🌟 The transmission coefficient and probability of a particle passing through a barrier are central concepts in quantum mechanics.
- 🔬 In a comparison between an electron and a proton with the same energy of 10 electron volts facing a 20 electron volt barrier, the proton's chances are significantly lower due to its greater mass.
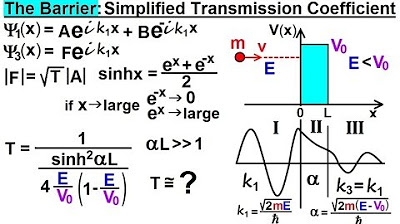
- 📉 The simplified transmission coefficient (T) can be approximated as T ≈ e^(-2αL), where α is the exponential decay constant and L is the barrier width.
- 🎯 α is calculated based on the formula α = √(2 * m * (V - E) / ħ), with m being the particle mass, V the barrier potential, E the particle energy, and ħ the reduced Planck constant.
- 🚀 For the proton, α is much larger due to its greater mass, leading to a smaller transmission coefficient and virtually zero probability of passing the barrier.
- 🌐 For the electron, a smaller α results in a larger transmission coefficient, indicating a 15.7% chance of passing through the barrier.
- 🔄 The barrier width (L) is a fixed number, and the main factor affecting the transmission coefficient is the particle's mass.
- 🤔 Larger particles, like alpha or beta particles in nuclear decay, require a higher energy to overcome the potential barrier and escape the nucleus.
- 💡 The difference in transmission probabilities highlights the particle's wave-like behavior in quantum mechanics, where smaller particles have a higher likelihood of tunneling through barriers.
- 🧠 Understanding these concepts is crucial for grasping nuclear decay processes and the behavior of particles in quantum states.
Q & A
What is the main topic of the transcript?
-The main topic of the transcript is the conceptual understanding of the transmission coefficient and the probability of a particle, specifically an electron and a proton, making it through a barrier with the same energy.
What are the key factors affecting the transmission coefficient?
-The key factors affecting the transmission coefficient are the energy of the particles, the potential of the barrier, the width of the barrier, and the mass of the particle.
How does the mass of a particle influence its probability of passing through a barrier?
-The mass of a particle significantly influences its probability of passing through a barrier. A heavier particle, like a proton, will have a larger exponential decay constant (alpha), resulting in a smaller transmission coefficient and thus a lower probability of passing through the barrier compared to a lighter particle like an electron.
What is the role of the exponential decay constant (alpha) in determining the transmission coefficient?
-The exponential decay constant (alpha) describes how the probability of a particle passing through a barrier declines as the barrier widens. A larger alpha results in a smaller transmission coefficient, indicating a lower probability of passage.
How is the potential barrier's width represented in the transmission coefficient formula?
-The potential barrier's width is represented as 'L' in the transmission coefficient formula, and it is a fixed number for a particular example. The wider the barrier, the smaller the probability of a particle passing through it.
What is the significance of the transmission coefficient in the context of nuclear decay?
-In the context of nuclear decay, the transmission coefficient is significant because it helps to determine how large particles, such as alpha or beta particles, can escape the nucleus. These particles need to have a very large energy to overcome the potential barrier and decay.
What happens to particles with energies insufficient to pass through the barrier?
-Particles with energies insufficient to pass through the barrier will be reflected off the barrier, or they may stop because their energy doesn't make it all the way through, resulting in partial penetration or no penetration at all.
How does the wavelength of a particle relate to its mass and the probability of passing through a barrier?
-A particle with a larger mass has a smaller wavelength, which makes it less likely to pass through a barrier due to the increased probability of interactions with the barrier's potential field.
What is the numerical value of the transmission coefficient for a proton with the given conditions?
-The numerical value of the transmission coefficient for a proton with the given conditions (10 eV energy, 20 eV barrier potential, and 100 picometers barrier width) is approximately equal to zero, indicating a very low probability of passing through the barrier.
What is the numerical value of the transmission coefficient for an electron under the same conditions?
-The numerical value of the transmission coefficient for an electron under the same conditions is approximately 0.157, or 15.7%, indicating a much higher probability of passing through the barrier compared to a proton.
How can the principles discussed in the transcript be applied to understanding particle behavior in quantum mechanics?
-The principles discussed in the transcript, such as the transmission coefficient and the effects of mass and energy on particle behavior, are fundamental to understanding quantum tunneling and the probabilistic nature of particle behavior in quantum mechanics.
Outlines
🔬 Quantum Mechanics: Electron vs Proton Barrier Transmission
This paragraph discusses the transmission coefficient and the probability of a particle, specifically an electron and a proton with the same energy, passing through a barrier. It introduces a simplified version of the transmission coefficient formula, highlighting the significance of the exponential decay constant (alpha) and how it varies with the mass of the particle. The comparison between the electron and proton's ability to pass through a barrier with a potential of 20 electron volts is made, emphasizing that the proton, having a larger mass, has a smaller transmission coefficient and thus a lower probability of making it through the barrier.
🌟 Particle Behavior in Nuclear Decay: Barrier Penetration
The second paragraph delves into the behavior of large particles during nuclear decay, explaining that they must overcome a potential barrier to be emitted from the nucleus. It contrasts the transmission probabilities of small and large particles, noting that smaller particles like electrons have a higher chance of penetrating the barrier, while larger particles such as protons require significantly more energy. The paragraph concludes by mentioning that future videos will provide examples of alpha and beta particles overcoming barriers, which is crucial for understanding nuclear decay processes.
Mindmap
Keywords
💡Transmission Coefficient
💡Electron Volts
💡Barrier
💡Potential Energy
💡Mass
💡Exponential Decay Constant (Alpha)
💡Wavelength
💡Quantum Tunneling
💡Probability
💡Nuclear Decay
💡Reflection
Highlights
The discussion compares the transmission of electrons and protons through a barrier with the same energy.
Both particles have an energy of 10 electron volts trying to pass through a 20 electron volt barrier.
The transmission coefficient is simplified for large alpha values, which is expected in this example.
The simplified transmission coefficient is approximately equal to e to the minus 2 alpha L, ignoring a small constant factor.
The alpha value is related to the mass of the particle; protons have a much larger alpha than electrons due to their greater mass.
A larger alpha results in a smaller transmission coefficient and thus a lower probability of passing through the barrier.
Protons are much less likely to pass through the barrier compared to electrons because of their larger mass and smaller wavelength.
The transmission coefficient for a proton is calculated to be almost zero, indicating very low probability.
For an electron, the transmission coefficient is significantly higher, with a 15.7% chance of passing through the barrier.
The example illustrates the impact of particle mass on the probability of quantum tunneling.
Larger particles require more energy to overcome the potential barrier in nuclear decay.
Alpha particles or beta particles need a large energy to penetrate the nuclear barrier.
The video aims to show examples of particles overcoming the nuclear barrier in future content.
The difference in transmission probability between protons and electrons demonstrates the principle of quantum tunneling.
Most particles will be reflected or stopped by the barrier, with only a small percentage making it through.
The content provides a conceptual understanding of the transmission coefficient and quantum tunneling probabilities.
Transcripts
Browse More Related Video
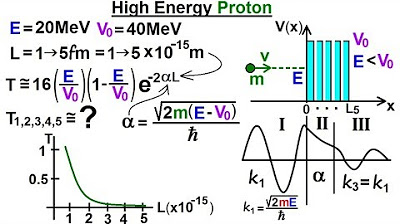
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (90 of 92) A High Energy Proton
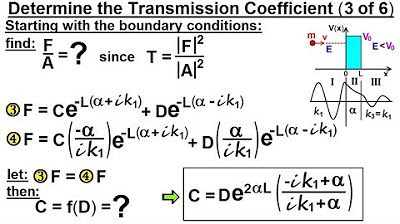
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (85 of 92) Transmission Coeff=? (3 of 6)
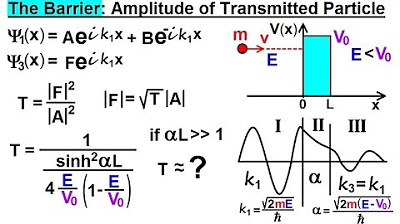
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (78 of 92) The Barrier: Amplitude
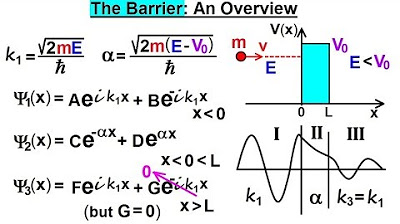
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (77 of 92) The Barrier: An Overview
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (79 of 92) Simplified Transmission Coeff.
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (76 of 92) Penetration Depth V0, E: Ex.
5.0 / 5 (0 votes)
Thanks for rating: