Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (42 of 92) k=? of a Diatomic Molecule
TLDRThis video delves into the spring constant of a diatomic molecule, using carbon monoxide as a case study. It explains how the molecule's vibrational frequency, observed through infrared radiation, can be related to its reduced mass and the spring constant. By calculating the energy absorbed or emitted in electron volts and applying quantum mechanics principles, the video demonstrates how the molecule's oscillatory behavior can be quantified, revealing that carbon monoxide vibrates at an astonishing 65 trillion times per second. Ultimately, it shows that the spring constant for such a molecule is approximately 2,000 Newtons per meter, highlighting the quantum jumps in energy during molecular absorption and emission processes.
Takeaways
- 🌟 The spring constant of a diatomic molecule is explored using carbon monoxide (CO) as an example.
- 📈 Carbon monoxide absorbs or emits radiation with an energy frequency of 0.27 electron volts, which is in the infrared range.
- 🔵 The energy of a photon (E) is related to Planck's constant (h) and the frequency (ν) of the photon: E = hν.
- 🎆 The frequency (ν) can be expressed as ν = Ω / 2π, where Ω is the angular frequency.
- 📊 The angular frequency (Ω) for CO is calculated using the observed energy (0.27 eV) and Planck's constant (h-bar).
- 🧬 The vibrational frequency of a CO molecule is found to be 65 trillion vibrations per second.
- 🔗 The reduced mass (μ) of the CO molecule is used to calculate the spring constant (K) as K = Ω^2 * μ.
- 📈 The calculated spring constant for CO is 1926 Newtons per meter, which is close to the observed value.
- 🌐 The units for the spring constant are derived from the combination of mass, length, and force units (kg m/s² becomes N/m).
- 🚀 Diatomic molecules like CO vibrate at high frequencies, with each vibration corresponding to a quantum energy level.
- 🌈 The energy difference (ΔE) between levels is quantified by h-bar times the angular frequency (h-barΩ), which is equal to 0.27 eV.
Q & A
What is the subject of the video?
-The video explores the spring constant of a diatomic molecule, specifically using carbon monoxide as an example.
What type of molecule is used as an example in the video?
-Carbon monoxide, which consists of a single carbon atom and a single oxygen atom, is used as the example diatomic molecule.
What is the energy frequency of the radiation absorbed or emitted by a carbon monoxide molecule?
-The energy frequency of the radiation absorbed or emitted by a carbon monoxide molecule is 0.27 electron volts, which is in the infrared range below the visible light range.
How is the energy of a photon related to its frequency?
-The energy of a photon is related to its frequency by the equation E = h * f, where E is the energy, h is Planck's constant, and f is the frequency of the photon.
What is the relationship between the angular frequency (Omega) and the reduced mass (μ) in the context of a diatomic molecule?
-The angular frequency (Omega) is related to the reduced mass (μ) through the equation Omega = sqrt(K/μ), where K is the spring constant and Omega is the angular frequency of the molecule's oscillation.
How many oscillations per second does a carbon monoxide molecule vibrate at?
-A carbon monoxide molecule vibrates at 65 trillion oscillations per second, which is its base vibration frequency.
What is the spring constant equivalent for a diatomic molecule like carbon monoxide?
-The spring constant equivalent for a diatomic molecule like carbon monoxide is measured to be 1926 Newtons per meter.
How can the spring constant be calculated from the observed vibrational frequency of a molecule?
-The spring constant can be calculated using the equation K = Omega^2 * μ, where Omega is the angular frequency of the molecule's oscillation and μ is the reduced mass.
What is the significance of the energy difference (ΔE) when a molecule absorbs or emits a photon?
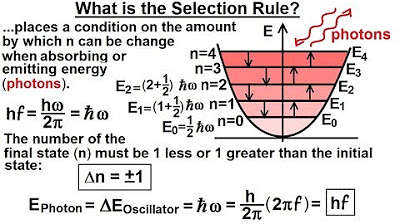
-The energy difference (ΔE) represents the amount of energy the molecule absorbs or emits as it jumps from one energy level to another. This energy change is quantized, meaning it occurs in discrete amounts equal to h-bar times the angular frequency (h-bar * Omega).
How does the concept of quantum jumps relate to the energy absorption and emission of a diatomic molecule?
-Quantum jumps describe the discrete changes in energy levels of a quantum mechanical oscillator, such as a diatomic molecule. The molecule can only absorb or emit energy in amounts equal to the energy of the photons associated with the difference in energy levels.
What is the practical implication of understanding the spring constant and vibrational frequency of diatomic molecules?
-Understanding the spring constant and vibrational frequency of diatomic molecules allows for the prediction and analysis of their behavior, including how they interact with electromagnetic radiation and their potential energy states, which is crucial in fields such as spectroscopy and molecular physics.
Outlines
🌟 Introduction to Diatomic Molecule's Spring Constant
This paragraph introduces the concept of the spring constant of a diatomic molecule, specifically focusing on carbon monoxide (CO). It explains that the energy frequency of radiation absorbed or emitted by CO lies in the infrared range, below visible light. The energy of a photon is described as being equal to Planck's constant times the frequency of the photon, which is then related to the spring constant through the vibrational frequency and reduced mass of the molecule. The paragraph concludes by calculating the spring constant for CO to be approximately 1926 Newtons per meter, which is close to the observed value.
Mindmap
Keywords
💡diatomic molecule
💡spring constant
💡Planck's constant
💡angular frequency
💡reduced mass
💡quantum jumps
💡infrared range
💡vibrational frequency
💡energy levels
💡oscillator
💡photon
Highlights
Exploration of the spring constant of a diatomic molecule, specifically carbon monoxide.
Carbon monoxide's energy frequency of radiation is in the infrared range, below visible light.
The energy absorbed or emitted by carbon monoxide is 0.27 electron volts.
The energy of a photon is calculated using Planck's constant times the frequency of the photon.
The basic frequency of a molecule is denoted as Omega sub nought or F sub nought.
The angular frequency (Omega) is calculated as HF divided by H bar.
The angular frequency of a carbon dioxide molecule is 4.1 x 10^14 oscillations per second.
The actual oscillations per second are found by dividing the angular frequency by 2 pi.
Carbon monoxide molecules vibrate at 65 trillion vibrations per second as their base vibration.
The spring constant for a diatomic molecule like carbon monoxide is derived from the reduced mass equation.
The spring constant is equivalent to 1926 Newtons per meter for carbon monoxide.
The equation for the spring constant is the square root of K over m M (reduced mass).
The reduced mass for carbon monoxide was calculated in a previous video.
The energy difference (delta energy) between levels is given by h-bar Omega.
The energy absorbed or emitted by the molecule is quantized, jumping from one level to another.
The molecule absorbs or emits photons equal to the energy difference between levels.
The relationship between the absorption and emission of energy by a diatomic molecule is explained through quantum mechanics.
A diatomic molecule like carbon monoxide acts as a quantum mechanical oscillator.
The spring constant of a typical diatomic molecule is in the order of 2,000 Newtons per meter.
The vibrational frequency of a typical diatomic molecule is in the order of many trillions of vibrations per second.
Transcripts
Browse More Related Video
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (51 of 92) Oscillator Amplitude - Diatomic
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (39 of 92) What is the Quantum Oscillator?
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (46 of 92) Quantum Nature of Oscillator 2
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (40 of 92) What is Reduced Mass?

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (50 of 92) What is Oscillator Amplitude?
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (44 of 92) What is the Selective Rule?
5.0 / 5 (0 votes)
Thanks for rating: