Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (50 of 92) What is Oscillator Amplitude?
TLDRThe lecture discusses the oscillation amplitude of diatomic molecules, using carbon monoxide as a case study. It explains how the amplitude varies with quantum energy levels and compares it to the equilibrium bond length. The lecture covers the calculation of vibrational frequency, zero-point energy, and energy transitions between quantum states. It also illustrates how the amplitude increases with energy levels, providing specific percentages relative to the bond length at different quantum states.
Takeaways
- 📌 The lecture focuses on analyzing the oscillation amplitude of diatomic molecules, specifically carbon monoxide (CO).
- 🔍 The equilibrium bond length between carbon and oxygen in CO is 112.8 picometers or 0.113 nanometers.
- 💫 Even at absolute zero (0 Kelvin), molecules vibrate due to quantum mechanical energy, with an amplitude of 1/2 Hbar Omega.
- 📈 The frequency of oscillation can be calculated using the formula √(K/m), resulting in 4.1 x 10^14 oscillations per second for CO.
- ⚡ The zero-point energy is approximately 4 x 10^-20 Joules, which corresponds to 0.135 electron volts.
- 🌐 Energy transitions between quantum states result in amplitude changes, doubling the energy results in a doubled amplitude.
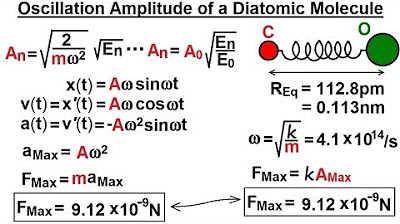
- 📊 The amplitude at any quantum level can be calculated using the formula √(2/K) * (MΩ)^2 * √(n), where n is the quantum number.
- 🔎 The amplitude ratio at different energy levels compared to the zero energy level is the square root of the energy level ratio.
- 🔢 At quantum levels 0, 1, 2, and 10, the energy and amplitude increase, with amplitudes being 0.42%, 7.3%, 9.4%, and 19.2% of the total bond distance, respectively.
- 🤔 The reduced mass used in calculations is the product of the masses of carbon and oxygen atoms divided by their sum, providing an equivalent mass for the oscillator model.
Q & A
What is the main topic of the lecture?
-The main topic of the lecture is to determine and understand the oscillation amplitude of a diatomic molecule, specifically carbon monoxide (CO), at various quantum energy levels and to compare it with the typical bond length between the two atoms.
What is the equilibrium bond length of the carbon monoxide molecule?
-The equilibrium bond length of the carbon monoxide molecule is 112.8 picometers, which is equivalent to 0.113 nanometers.
How does the molecule's vibration change at absolute zero Kelvin?
-Even at absolute zero Kelvin, the molecule will still vibrate with the quantum mechanical energy it possesses, which is 1/2 Hbar Omega.
What is the frequency of oscillation for the carbon monoxide molecule?
-The frequency of oscillation for the carbon monoxide molecule is the square root of (K/m), which is approximately 4.1 times 10^14 oscillations per second.
How is the energy at the zero energy level calculated?
-The energy at the zero energy level (E_sub_nought) is calculated to be about 4 times 10^-20 joules, which is equivalent to 0.135 electron volts when converted.
What is the formula to calculate the amplitude at any quantum level?
-The amplitude at any quantum level (n) can be calculated using the formula: amplitude = sqrt(2/K) * M * Omega^2 * sqrt(E_n), where E_n is the energy at that quantum level.
How does the amplitude change as the quantum energy level increases?
-As the quantum energy level increases, the amplitude increases proportionally to the square root of the energy level. For example, at the first quantum level (N=1), the amplitude increases by the square root of 3, and at the fifth quantum level (N=5), the amplitude is 2.5 times that of the first level.
What is the percentage of the amplitude relative to the bond length at the zero energy level?
-At the zero energy level, the amplitude is 4.7 picometers, which is 4.2% of the total bond length between the two atoms.
How does the reduced mass affect the oscillator model?
-The reduced mass, which is the product of the masses of the carbon and oxygen atoms divided by the sum of the two, is used in the oscillator model to determine the equivalent mass that influences the oscillation properties of the carbon monoxide molecule.
What is the significance of understanding the oscillation amplitude at different quantum states?
-Understanding the oscillation amplitude at different quantum states provides a realistic picture of molecular vibrations and helps in predicting the behavior of molecules under various energy conditions, which is crucial for studying chemical bonding and molecular properties.
Outlines
📚 Introduction to Diatomic Molecule Oscillation
This paragraph introduces the topic of diatomic molecule oscillation, focusing on the carbon monoxide (CO) molecule. It discusses the aim to determine the oscillation amplitude at various quantum energy levels and compares these to the typical bond length of the molecule. The equilibrium bond length of CO is given as 112.8 picometers or 1.13 nanometers. The paragraph sets the stage for exploring how the molecule's oscillation amplitude changes as it gains energy, even at absolute zero temperature, where it still vibrates with quantum mechanical energy.
Mindmap
Keywords
💡Diatomic molecule
💡Oscillation amplitude
💡Quantum energy levels
💡Bond length
💡Equilibrium position
💡Zero-point energy
💡Frequency
💡Reduced mass
💡Energy quantization
💡Amplitude ratio
Highlights
The lecture focuses on diatomic molecules and their oscillation amplitude at various quantum energy levels.
Carbon monoxide (CO) molecule is used as an example, consisting of one carbon and one oxygen atom.
The equilibrium bond length for CO is 112.8 picometers or 0.113 nanometers.
Even at absolute zero (0 Kelvin), molecules still vibrate due to quantum mechanical energy.
The frequency of oscillation for CO can be calculated using the formula √(K/m), resulting in 4.1 x 10^14 oscillations per second.
The zero energy level (E_sub_0) has an amplitude of 4.7 picometers, which is 4.2% of the total bond distance.
The amplitude increases with the quantum energy level; for n=1, the amplitude is about 8.2 picometers (7.3% of the bond distance).
At the fifth quantum level (n=5), the amplitude is approximately 9.4% of the total bond distance.
At the tenth quantum level (n=10), the amplitude reaches 21.7 picometers, which is 19.2% of the total bond distance.
The reduced mass used in calculations is the product of the carbon and oxygen atom masses divided by their sum.
The lecture provides a realistic scenario for understanding the oscillation amplitude of a real molecule like CO.
The oscillation amplitude is directly related to the quantum energy level of the molecule.
The energy jumps between quantum states are quantified in electron volts, with the zero energy level having 0.135 eV.
The energy at higher quantum levels can be calculated using the ratio of energy levels and amplitudes.
The lecture demonstrates the physical feel of oscillation amplitude in relation to the bond distance of diatomic molecules.
The theoretical contributions include the use of quantum mechanics to understand molecular vibrations.
Practical applications may involve using these insights to predict molecular behavior under different energy conditions.
Transcripts
Browse More Related Video
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (48 of 92) How Oscillator Increases energy

5. Resonance V and Atoms I
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (39 of 92) What is the Quantum Oscillator?
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (51 of 92) Oscillator Amplitude - Diatomic
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (42 of 92) k=? of a Diatomic Molecule
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (46 of 92) Quantum Nature of Oscillator 2
5.0 / 5 (0 votes)
Thanks for rating: