Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (51 of 92) Oscillator Amplitude - Diatomic
TLDRThe video script delves into the comparison between a quantum-mechanical oscillator and a classical mechanical oscillator, using the example of a carbon monoxide molecule. It explains how quantum mechanics equations must align with classical mechanics, detailing the molecule's spring constant, amplitude at the zero energy level, reduced mass, and oscillation frequency. The script then applies classical mechanics formulas to determine the position, velocity, and acceleration of the oscillator, ultimately showing that quantum-mechanical oscillators adhere to Newton's second law and the principles of classical mechanical oscillators, providing insights into the behavior of particles at the quantum level.
Takeaways
- 🌟 Quantum-mechanical oscillators can be compared to classical mechanical oscillators to understand their behavior.
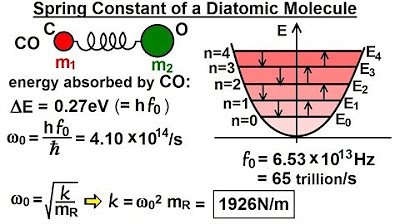
- 📐 The spring constant for the carbon monoxide molecule is 1926 Newtons per meter.
- 📍 The amplitude at the zero energy level (n=0) in the quantum mechanical stage is 4.7×10^-12 meters.
- 🔄 The oscillation frequency is 4.1×10^14 radians per second, not oscillations per second.
- 📈 The position of a classical oscillator over time can be described by amplitude times sine or cosine of Omega T.
- 🚀 The velocity of a classical oscillator is given by Omega times cosine of Omega T.
- 💥 The maximum acceleration of a quantum mechanical Slater is 7.96×10^17 meters per second squared.
- 🔧 Newton's second law (F=MA) applies to quantum mechanical oscillators, relating force to mass and acceleration.
- 🌐 The maximum force experienced by a quantum mechanical oscillator is due to intermolecular forces at the maximum amplitude of oscillation.
- 🔢 The spring constant derived from the quantum mechanical model matches the classical mechanical spring constant when comparing maximum forces.
- 🎯 The quantum mechanical oscillator follows the same rules and regulations as the classical mechanical oscillator, as shown by the consistency in force and acceleration calculations.
Q & A
What is the purpose of comparing a quantum-mechanical oscillator to a classical mechanical oscillator?
-The purpose is to demonstrate that quantum mechanical equations must match classical mechanical predictions in order to be considered correct, thereby showing the connection and consistency between quantum and classical mechanics.
What was the spring constant determined for the carbon monoxide molecule in the discussion?
-The spring constant for the carbon monoxide molecule was determined to be 1926 Newtons per meter.
What is the amplitude at the zero energy level in the quantum mechanical model?
-At the zero energy level (n=0), the amplitude is 4.7 times 10 to the minus 12 meters.
What is the reduced mass calculated for the oscillation?
-The reduced mass for the oscillation was calculated to be 1.1 times 10 to the minus 26 kilograms.
What is the oscillation frequency of the carbon monoxide molecule?
-The oscillation frequency is 4.1 times 10 to the 14 radians per second.
How is the position of a classical mechanical oscillator expressed as a function of time?
-The position of a classical mechanical oscillator is expressed as the amplitude times the sine or cosine of Omega T.
What is the relationship between the maximum amplitude and the energy state of the quantum mechanical oscillator?
-The maximum amplitude of the quantum mechanical oscillator is related to the energy state through the equation a Max = a * Omega squared, where 'a' is the amplitude and Omega is the angular frequency.
How does Newton's second law relate to the force experienced by a quantum mechanical oscillator?
-According to Newton's second law (F=MA), the force experienced by a quantum mechanical oscillator is equal to the mass times the acceleration, which can be calculated using the spring constant and the maximum amplitude.
What is the maximum force experienced by the carbon monoxide molecule at the maximum amplitude of oscillation?
-The maximum force experienced at the maximum amplitude of oscillation is 9.12 times 10 to the minus 9 Newtons.
How does the maximum force relate to the spring constant and the amplitude?
-The maximum force is calculated by multiplying the spring constant (K) by the maximum amplitude (A), which in this case confirms the consistency between quantum mechanics and classical mechanics.
What conclusion can be drawn from the analysis of the quantum mechanical oscillator in relation to Newton's laws and classical mechanics?
-The analysis shows that even the quantum mechanical oscillator follows the same rules and regulations as described by Newton's second law and the properties of a classical mechanical oscillator, thus bridging the gap between quantum and classical descriptions of physical systems.
Outlines
📐 Quantum-Mechanical Oscillator Comparison
This paragraph introduces the concept of a quantum-mechanical oscillator by comparing it to a classical mechanical oscillator. It discusses the carbon monoxide molecule's spring constant, amplitude at the zero energy level, reduced mass, and oscillation frequency. The paragraph explains how the position, velocity, and acceleration of an oscillator can be described using classical mechanics equations. It then calculates the maximum acceleration and force experienced by the quantum-mechanical oscillator at its maximum amplitude, demonstrating that quantum-mechanical oscillators follow Newton's second law and the rules of classical mechanical oscillators.
Mindmap
Keywords
💡Quantum-mechanical Slayer
💡Classical mechanical oscillator
💡Spring constant
💡Amplitude
💡Reduced mass
💡Oscillation frequency
💡Angular frequency (Omega)
💡Maxwell's equations
💡Newton's second law (F=ma)
💡Intermolecular forces
💡Energy levels
💡Total distance between atoms
Highlights
Comparing a quantum-mechanical oscillator to a classical mechanical oscillator to understand the similarities and differences.
The spring constant of the carbon monoxide molecule is 1926 Newtons per meter.
The amplitude at the zero energy level in the quantum mechanical stage is 4.7 x 10^-12 meters.
The reduced mass for the oscillation frequency is 1.1 x 10^-26 kilograms.
The oscillation frequency is 4.1 x 10^14 radians per second.
The position of a classical oscillator as a function of time can be expressed as the amplitude times the sine or cosine of Omega T.
The velocity of a classical oscillator is given by Omega times the cosine of Omega T.
The maximum acceleration of a quantum mechanical oscillator is calculated to be 7.96 x 10^17 meters per second squared.
Newton's second law (F=MA) is used to calculate the force on the quantum mechanical oscillator.
The maximum force experienced by the spring is due to intermolecular forces at the maximum amplitude.
The force calculated using the spring constant matches the force calculated using Newton's second law.
Quantum mechanical oscillators follow the same rules and regulations as classical mechanical oscillators.
The maximum force (F_max) is experienced when the spring is pulled back to its maximum amplitude.
The analysis provides a good understanding of quantum mechanical oscillators and their energy levels.
The percentage of the total distance between atoms is considered when evaluating the oscillation amplitudes.
The transcript explains the mathematical and physical principles behind quantum and classical oscillators in detail.
Transcripts
Browse More Related Video
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (48 of 92) How Oscillator Increases energy

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (38 of 92) Simple Harmonic Oscillator
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (40 of 92) What is Reduced Mass?
Advanced Quantum Mechanics Lecture 3
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (44 of 92) What is the Selective Rule?
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (42 of 92) k=? of a Diatomic Molecule
5.0 / 5 (0 votes)
Thanks for rating: