Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (44 of 92) What is the Selective Rule?
TLDRThe video script delves into the concept of selection rules in quantum mechanics, focusing on the behavior of quantum mechanical oscillators. It explains that these oscillators can gain or lose energy by emitting or absorbing photons, and that the energy difference between states is quantified by the Planck's constant times the angular frequency of oscillation. The selection rule dictates that the change in quantum number (Δn) when absorbing or emitting energy can only be by one, meaning the oscillator can only transition to an adjacent energy state. This principle is key to understanding the quantization of energy levels and the energy exchange involving photons, as established by the photoelectric effect.
Takeaways
- 📌 Quantum mechanical oscillators can gain or lose energy by emitting or absorbing photons.
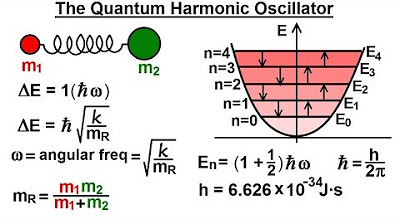
- 🔧 The lowest energy state of an oscillator is not zero, but 1/2 Hbar times Omega, where Omega is the angular frequency of oscillation.
- ⚡ The energy difference between consecutive quantum states is exactly one Hbar times Omega.
- 🚫 The selection rule imposes a condition on how much the quantum number (n) can change when energy is absorbed or emitted, which must be by one unit.
- 🌟 The final state's quantum number must be one less or one greater than the initial state after a photon is absorbed or emitted.
- 🔄 The oscillator can only transition between adjacent energy levels, not jumping over multiple levels at once.
- 💰 The energy of the photon involved in the transition is equal to the energy difference between the two adjacent states.
- 🎢 The harmonic oscillator can only increase or decrease by one level at a time, reflecting the discrete nature of energy changes.
- 🌐 The energy of the photon (E) is related to the frequency (F) by the equation E = h * F, derived from the photoelectric effect.
- 📈 The selection rule is fundamental for understanding quantum transitions and the energy exchange in quantum systems.
Q & A
What is the selection rule in quantum mechanics?
-The selection rule in quantum mechanics is a condition that dictates the amount by which the quantum number (n) can change when a quantum mechanical oscillator absorbs or emits energy, typically in the form of photons.
How does the selection rule relate to the energy levels of a quantum mechanical oscillator?
-The selection rule states that the energy difference between two adjacent energy levels of a quantum mechanical oscillator is equal to the energy of a single photon absorbed or emitted, which means the quantum number can only change by one (Δn = ±1).
What is the significance of the ground state energy level in a quantum mechanical oscillator?
-The ground state energy level of a quantum mechanical oscillator is significant because it is not equal to zero; it is 1/2 ħω, where ω is the angular frequency of the oscillation. This indicates that there is a minimum energy state that the oscillator cannot fall below.
What is the relationship between the energy levels and the frequency of oscillation in a quantum mechanical oscillator?
-The energy difference between consecutive energy levels in a quantum mechanical oscillator is directly related to the frequency of oscillation (ω). The energy difference is given by ħω, where ħ is the reduced Planck's constant.
How does the selection rule limit the possible transitions for a quantum mechanical oscillator?
-The selection rule limits the possible transitions of a quantum mechanical oscillator by stating that the oscillator can only transition to an energy level that is one less or one greater than its current state, meaning it can only absorb or emit photons with energy equal to the energy difference between adjacent levels.
What is the basic unit of energy for a photon?
-The basic unit of energy for a photon is given by the equation E = hν, where E is the energy, h is Planck's constant, and ν is the frequency of the photon. This relationship was established by the photoelectric effect.
How does the selection rule ensure that the energy of the emitted or absorbed photon matches the energy difference between two states?
-The selection rule ensures this by stating that the quantum number change (Δn) must be ±1, which corresponds to the transition between adjacent energy levels. Since the energy difference between these levels is ħω, the energy of the emitted or absorbed photon must be ħω, matching the energy difference.
What is the consequence of the selection rule for the harmonic oscillator's energy transitions?
-The consequence of the selection rule is that the harmonic oscillator can only transition one level at a time, either increasing or decreasing its energy by the amount equivalent to the energy difference between two adjacent states.
How does the selection rule relate to the photoelectric effect?
-The selection rule is related to the photoelectric effect because it confirms that the energy of a photon is equal to the energy difference between two states (ħω), which is the basic unit of photon energy as discovered by the photoelectric effect.
What happens when a quantum mechanical oscillator absorbs or emits a photon?
-When a quantum mechanical oscillator absorbs a photon, it gains energy and transitions to a higher energy state, while emitting a photon results in the loss of energy and a transition to a lower energy state, both following the selection rule with Δn = ±1.
Can a quantum mechanical oscillator jump two or more states at once according to the selection rule?
-No, according to the selection rule, a quantum mechanical oscillator can only change by one state at a time, as the energy difference between non-adjacent states is not equal to the energy of a single photon.
Outlines
📚 Introduction to Quantum Mechanics Oscillator and Selection Rule
This paragraph introduces the concept of the selection rule in quantum mechanics, specifically in relation to quantum mechanical oscillators. It explains that these oscillators can gain or lose energy by emitting or absorbing photons, and that the energy levels are quantized, with the lowest energy state not being zero but rather 1/2 H-bar times Omega. The selection rule is then defined, stating that the change in quantum number (n) when absorbing or emitting energy can only be by one unit, meaning the final state's quantum number must be one less or one greater than the initial state. This is directly related to the energy difference between adjacent quantum states, which is quantified as H-bar times Omega. The explanation concludes by discussing the implications of this rule, emphasizing that the oscillator can only change energy by one level at a time, and the energy of the photon involved in the process is equal to the energy difference between two adjacent states.
Mindmap
Keywords
💡Quantum Mechanics
💡Oscillator
💡Selection Rule
💡Energy Level
💡Photon
💡h-bar
💡Omega
💡Quantum Number (n)
💡Energy Transition
💡Harmonic Oscillator
💡Photoelectric Effect
Highlights
Exploration of the selection rule, a key property in quantum mechanics related to oscillators.
Quantum mechanical oscillators gain or lose energy through the emission or absorption of photons.
The lowest energy level of an oscillator is 1/2 H-bar times Omega, which is not zero.
The energy difference between consecutive quantum states is exactly one H-bar times Omega.
The selection rule places a condition on how much the quantum number (n) can change during energy absorption or emission.
The change in the quantum number (Delta n) can only be one, meaning the oscillator can only absorb or emit energy in single photon increments.
The final state's quantum number must be one less or one greater than the initial state after photon absorption or emission.
The energy of the photon involved in the process is equal to the energy difference between two adjacent states of the oscillator.
The oscillator can only change by one level at a time, not by two or more simultaneously.
The energy absorbed or emitted is equal to the photon's energy difference between two adjacent levels.
The selection rule is fundamental for understanding the behavior of quantum oscillators and their interaction with photons.
The concept of H-bar (H over 2 pi) and Omega (angular frequency) are introduced to explain the energy quantization.
The energy of a photon is equal to h times the frequency, as discovered by the photoelectric effect.
The selection rule is a specific limit that dictates the quantized nature of energy changes in quantum oscillators.
The lecture provides a detailed explanation of the quantum mechanical oscillator's energy levels and the implications of the selection rule.
Transcripts
Browse More Related Video
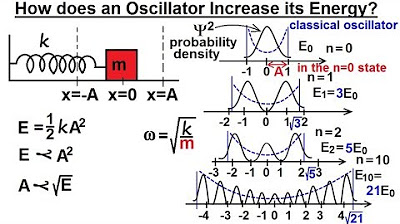
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (48 of 92) How Oscillator Increases energy
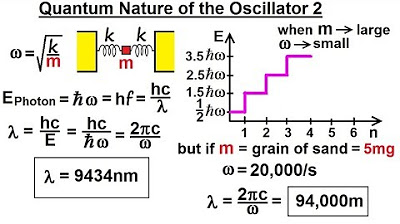
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (46 of 92) Quantum Nature of Oscillator 2

Advanced Quantum Mechanics Lecture 4
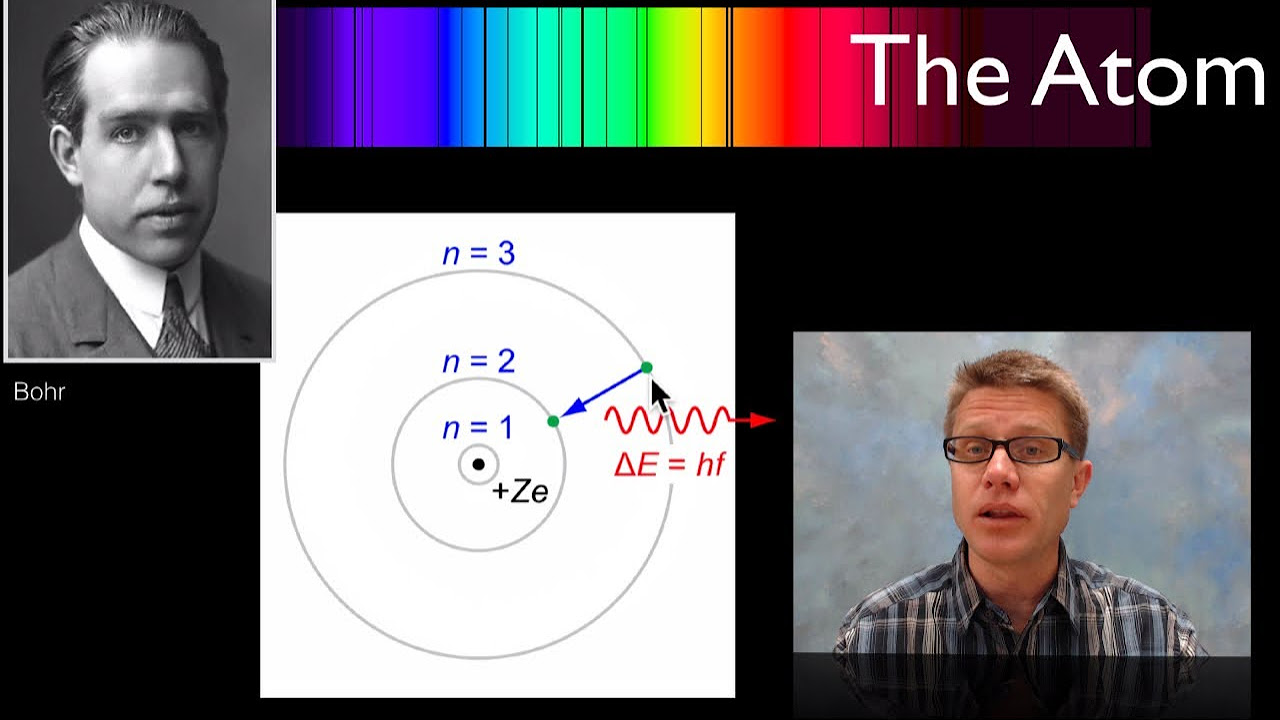
The Bohr Atom
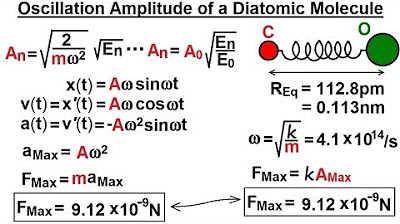
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (51 of 92) Oscillator Amplitude - Diatomic
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (39 of 92) What is the Quantum Oscillator?
5.0 / 5 (0 votes)
Thanks for rating: