Chiral vs Achiral Molecules - Chirality Carbon Centers, Stereoisomers, Enantiomers, & Meso Compounds
TLDRThis video script offers a comprehensive guide on identifying chiral centers in molecules, determining the number of stereoisomers, and distinguishing between chiral and achiral molecules. It explains the concept of chiral centers as carbon atoms with four distinct groups and uses examples to illustrate how to calculate potential stereoisomers (up to 2^n, where n is the number of chiral centers). The script also clarifies the difference between enantiomers, which are non-superposable mirror images, and meso compounds, which have an internal plane of symmetry, providing a clear understanding of molecular stereochemistry.
Takeaways
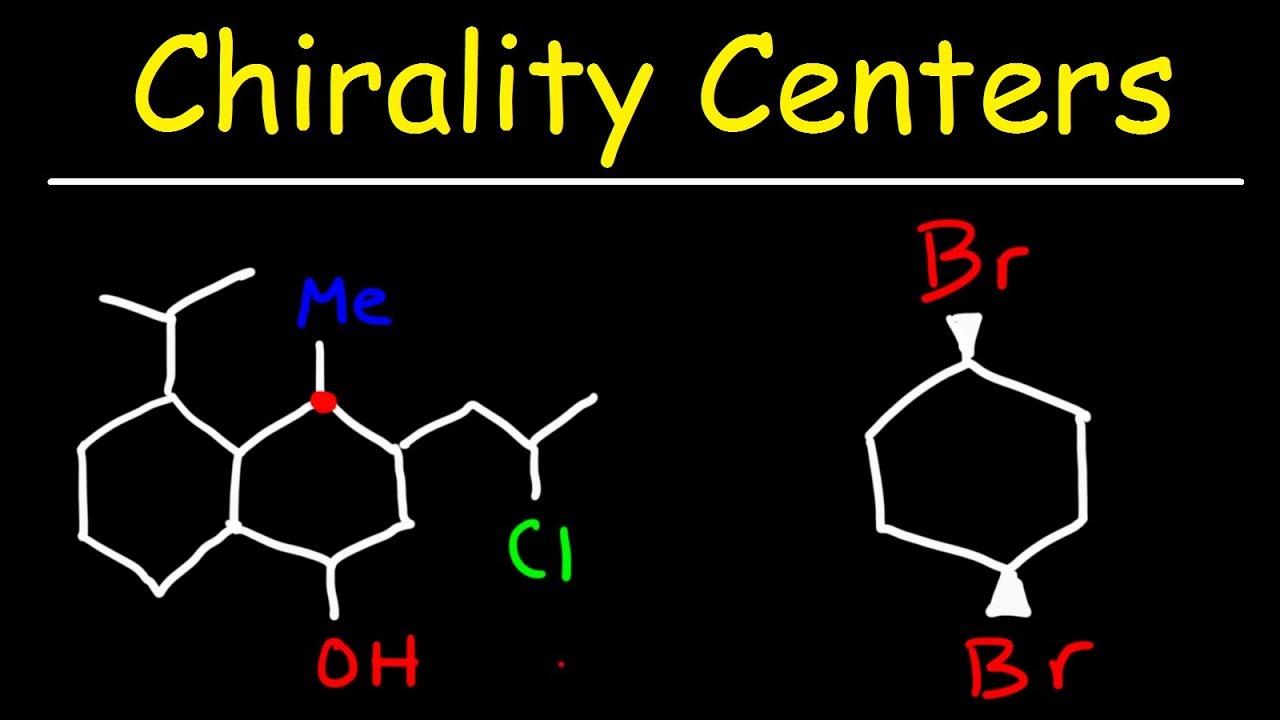
- 🧬 A chiral center, also known as an asymmetric carbon, is a carbon atom bonded to four different groups.
- 🔍 Primary and secondary carbons are not chiral because they do not have four distinct groups attached.
- 🤔 Even a tertiary carbon with only one hydrogen can be non-chiral if it has two identical groups attached.
- 🔑 The presence of a chiral center in a molecule indicates the potential for stereoisomerism.
- 🌐 The number of possible stereoisomers for a molecule is calculated as 2 to the power of the number of chiral centers (2^n).
- 🤹♂️ Stereoisomers have the same connectivity but differ in the spatial arrangement of their atoms.
- 🪞 Enantiomers are a type of stereoisomer that are mirror images of each other and are non-superposable.
- 🔄 The configuration of each chiral center can be either R or S, contributing to the total number of stereoisomers.
- 📏 Achiral molecules have a line of symmetry or lack chiral centers, making them superposable on their mirror images.
- 🔄 Meso compounds are a special case of stereoisomers with an internal plane of symmetry, making them identical despite having chiral centers.
- 🔄 The presence of multiple chiral centers does not guarantee a chiral molecule; symmetry must also be considered.
Q & A
What is a chiral center in chemistry?
-A chiral center, also known as an asymmetric carbon, is a carbon atom that has four different groups attached to it, which makes it not superimposable on its mirror image.
How can you identify if a carbon is chiral based on the script?
-A carbon is chiral if it has four different groups attached to it. Primary carbons with three hydrogens and secondary carbons with two hydrogens are not chiral.
Why is a tertiary carbon with two ethyl groups not considered a chiral center?
-Even though it is a tertiary carbon, the presence of two identical ethyl groups means it does not have four different groups, hence it is not a chiral center.
What is the maximum number of stereoisomers for a molecule with one chiral center?
-For a molecule with one chiral center, the maximum number of stereoisomers is 2, as it can be either in the R or S configuration.
What is the relationship between the two stereoisomers shown in the script?
-The two stereoisomers are enantiomers, which are non-superposable mirror images of each other, similar to a left and right hand.
How can you determine if a molecule is chiral or achiral by looking at its structure?
-A molecule is chiral if it has at least one chiral center and lacks a line of symmetry. If it has a line of symmetry or no chiral centers, it is achiral.
What is the maximum number of stereoisomers for a molecule with three chiral centers?
-For a molecule with three chiral centers, the maximum number of stereoisomers is 2 to the power of 3, which equals 8.
What are the different configurations of a chiral center?
-Each chiral center can have a configuration of R or S, and these can combine in various ways to form different stereoisomers.
What is a meso compound in the context of stereochemistry?
-A meso compound is a type of stereoisomer that has an internal plane of symmetry, making it identical to its mirror image despite having chiral centers.
How can you distinguish between enantiomers and meso compounds?
-Enantiomers are non-superposable mirror images with all chiral centers having opposite configurations, while meso compounds have an internal plane of symmetry and are identical to their mirror image.
Outlines
🧬 Understanding Chirality and Stereoisomers
This paragraph introduces the concept of chiral centers and stereoisomers in chemistry. A chiral center, often an asymmetric carbon atom, has four different groups attached to it. The script explains how to identify chiral centers by examining the groups attached to carbon atoms, using examples of primary, secondary, and tertiary carbons. It clarifies that not all tertiary carbons are chiral, as shown with a carbon having two ethyl groups. The paragraph also discusses how to determine if a molecule is chiral or achiral and introduces the concept of stereoisomers, specifically enantiomers, which are non-superposable mirror images of each other.
📊 Counting Stereoisomers and Identifying Chiral Centers
The second paragraph delves deeper into the process of identifying chiral centers within a molecule and calculating the number of possible stereoisomers. It explains the formula for determining the maximum number of stereoisomers, which is 2 to the power of the number of chiral centers (n). Using examples, the script illustrates how to draw potential stereoisomers, emphasizing that they can be in different configurations (R or S). It also discusses the concept of meso compounds, which have an internal plane of symmetry, and distinguishes them from enantiomers, which lack symmetry and have all chiral centers in opposite configurations.
🔍 Analyzing Molecular Symmetry and Chirality
The final paragraph focuses on analyzing molecular symmetry to determine if a molecule is chiral or achiral. It provides examples of molecules with and without chiral centers and explains how the presence of a line of symmetry indicates an achiral molecule. The script also discusses how the configuration of chiral centers affects the overall symmetry of a molecule, using butane as an example of an achiral molecule due to its symmetry. It concludes with a comparison of different molecular relationships, such as meso compounds and enantiomers, highlighting the importance of symmetry and chiral center configurations in classifying molecules.
Mindmap
Keywords
💡Chiral Center
💡Stereoisomers
💡Chirality
💡Achirality
💡Enantiomers
💡Meso Compounds
💡Tertiary Carbon
💡Primary Carbon
💡Secondary Carbon
💡Configuration
💡Symmetry
Highlights
The video focuses on identifying chiral centers and determining the number of stereoisomers in a molecule.
A chiral center, also known as an asymmetric carbon, has four different groups attached.
Primary and secondary carbons are not chiral due to the presence of multiple hydrogens.
A tertiary carbon with two ethyl groups is not a chiral center despite having only one hydrogen.
A carbon with a bromine atom, a hydrogen, a methyl, and a propyl group is a chiral center.
The maximum number of stereoisomers is 2 to the power of the number of chiral centers.
Stereoisomers are isomers with the same connectivity but different spatial arrangements.
Enantiomers are non-superposable mirror images, like left and right hands.
A molecule with three chiral centers can have up to eight potential stereoisomers.
Chiral centers can have R or S configurations, leading to various stereoisomer combinations.
Butane is an achiral molecule due to its line of symmetry and lack of chiral centers.
A molecule with one chiral center is chiral if it lacks symmetry.
A molecule with two chiral centers can be achiral if it has a line of symmetry.
Meso compounds have an internal plane of symmetry and are identical.
Molecules with changed configurations of chiral centers and lack of symmetry are enantiomers.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: