7.4 Quantum Numbers | High School Chemistry
TLDRThis lesson explores quantum numbers, which uniquely identify an electron's position in an atom. Four quantum numbers are discussed: the principal (n), azimuthal (l), magnetic (m_l), and spin (m_s) numbers. These numbers define the electron's shell, subshell, orbital orientation, and spin state, respectively. The lesson also covers the Pauli Exclusion Principle, emphasizing that no two electrons in an atom can have the same set of four quantum numbers.
Takeaways
- 🌐 Quantum numbers are like addresses for electrons in an atom, helping to identify their specific location within the atom's orbitals.
- 🔢 There are four quantum numbers that uniquely define the state of an electron in an atom.
- 🚫 The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers.
- 📍 The principal quantum number (n) indicates the electron's shell or energy level, ranging from 1 to infinity.
- 📏 The azimuthal quantum number (l) identifies the subshell type (s, p, d, f), with values ranging from 0 to n-1.
- 🧭 The magnetic quantum number (m_l) determines the specific orbital within a subshell, with values from -l to +l.
- 🌀 The spin quantum number (m_s) represents the electron's spin, with only two possible values: +1/2 or -1/2.
- 🌟 Each quantum number provides a part of the 'code' for an electron's address, similar to a house, street, city, and state.
- 🚫 In an s subshell, the m_l value is always 0 because there is only one orbital, whereas other subshells have multiple orbitals.
- 🔑 Understanding the range of values for each quantum number is crucial for identifying an electron's specific location in an atom.
- 📚 The lesson is part of a high school chemistry playlist, with new lessons released weekly during the 2020-21 school year.
Q & A
What are quantum numbers and why are they important in understanding atomic structure?
-Quantum numbers are a set of four numerical values that define the state of an electron in an atom. They are crucial for determining the location and properties of electrons within the atom's orbitals, providing a unique 'address' or code for each electron.
What is the Pauli Exclusion Principle, and how does it relate to quantum numbers?
-The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This principle ensures that electrons occupy different energy states and prevents them from sharing the same exact orbital with the same spin.
How many quantum numbers are there, and what do they represent?
-There are four quantum numbers: n (principal quantum number), l (azimuthal quantum number), m_l (magnetic quantum number), and m_s (spin quantum number). They represent the electron's shell, subshell, specific orbital orientation, and spin state, respectively.
What does the principal quantum number (n) signify in terms of an electron's position?
-The principal quantum number (n) indicates the shell or energy level in which the electron resides. It ranges from 1 to infinity and corresponds to the distance of the electron from the nucleus.
What is the significance of the azimuthal quantum number (l) in identifying an electron's subshell?
-The azimuthal quantum number (l) determines the subshell (s, p, d, or f) in which the electron is located. It ranges from 0 to n-1, where each value corresponds to a specific subshell type.
How does the magnetic quantum number (m_l) relate to the orientation of an electron's orbital?
-The magnetic quantum number (m_l) identifies the specific orbital within a subshell. It takes integer values from -l to +l, indicating the orbital's orientation in space relative to the x, y, or z-axis.
What are the possible values for the spin quantum number (m_s), and what do they represent?
-The spin quantum number (m_s) can have two possible values: +1/2 or -1/2. These values represent the two possible spin states of an electron, often referred to as 'spin up' and 'spin down'.
Can two electrons occupy the same orbital with the same spin quantum number?
-No, according to the Pauli Exclusion Principle, two electrons cannot have the same set of quantum numbers, which includes the spin quantum number. Thus, they cannot occupy the same orbital with the same spin.
How do the values of the azimuthal quantum number (l) depend on the principal quantum number (n)?
-The values of l are dependent on n, as l can range from 0 to n-1. For example, if n=3, l can be 0, 1, or 2, corresponding to s, p, or d subshells, respectively.
What is the range of possible values for the magnetic quantum number (m_l) based on the azimuthal quantum number (l)?
-The range of values for m_l is determined by l, and it can take integer values from -l to +l. For instance, if l=1, m_l can be -1, 0, or +1.
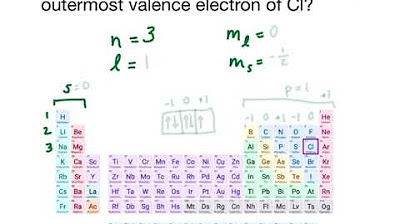
How can you identify a valence electron in an atom like sodium using quantum numbers?
-For a valence electron in sodium (atomic number 11), you know that the electron is in the third shell (n=3), in an s subshell (l=0), and m_l must be 0. The spin quantum number m_s can be either +1/2 or -1/2, so there are two possible sets of quantum numbers for the valence electron.
Outlines
🌐 Quantum Numbers: Electron's Address in an Atom
This paragraph introduces the concept of quantum numbers, which are essential in understanding the location and behavior of electrons within an atom. It explains that each electron has a unique set of four quantum numbers that act as an address, indicating its position in the atom. The video aims to define these quantum numbers and discuss their significance in identifying an electron's location. The narrator also mentions that the lesson is part of a high school chemistry playlist, encouraging viewers to subscribe and stay updated with new content.
🔍 Understanding Quantum Numbers: Principles and Values
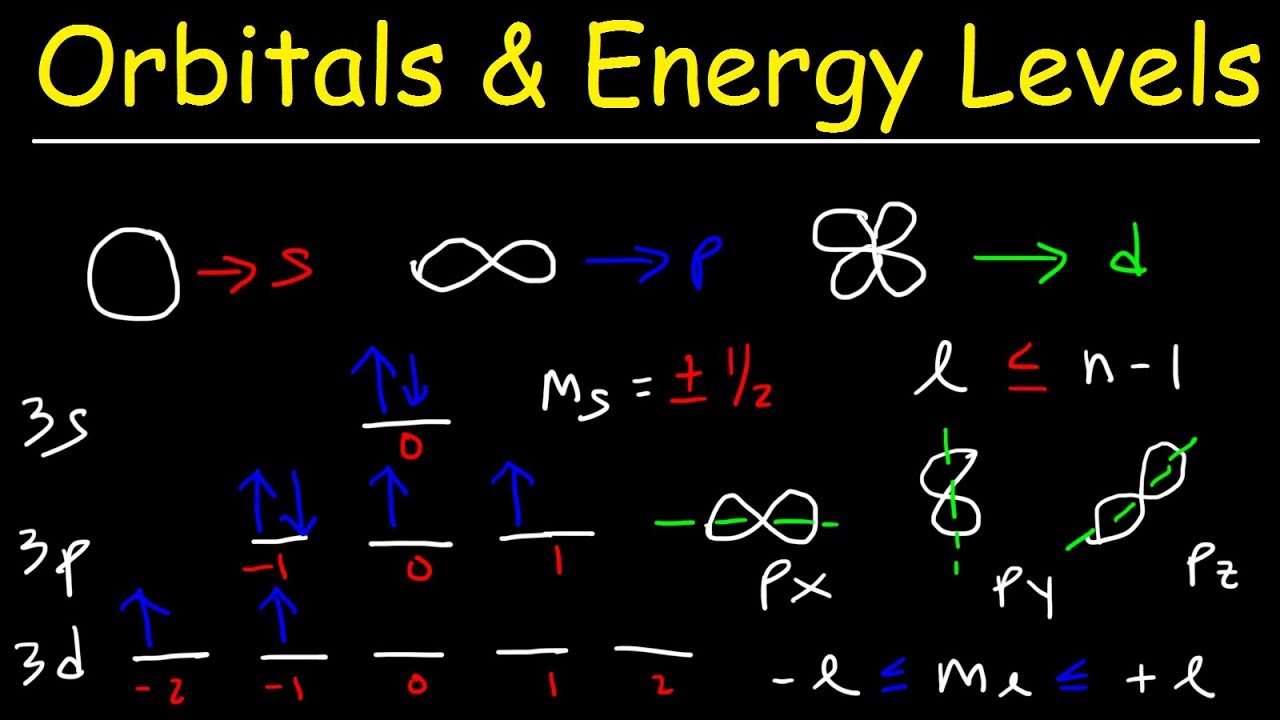
This paragraph delves deeper into the specifics of quantum numbers. It discusses the principal quantum number (n), which determines the electron's shell or energy level. The azimuthal quantum number (l), also known as the angular momentum quantum number, identifies the subshell type (s, p, d, or f). The magnetic quantum number (m_l) specifies the orientation of the orbital in space, and the spin quantum number (m_s) indicates the electron's spin direction. The paragraph also explains the range of values for these quantum numbers, emphasizing that no two electrons can share the same set of four quantum numbers due to the Pauli Exclusion Principle.
🌟 Quantum Numbers in Practice: Identifying Electrons
The final paragraph focuses on applying the concept of quantum numbers to identify specific electrons in an atom. It uses the example of a valence electron in sodium to illustrate how to determine a set of possible quantum numbers. The narrator explains that while some quantum numbers can be uniquely determined (e.g., n and l for an s subshell), others like m_s can only be known to be either +1/2 or -1/2. The paragraph concludes with an invitation for viewers to engage with the content by liking, sharing, and exploring additional resources such as study guides and practice problems available on the instructor's website.
Mindmap
Keywords
💡Quantum Numbers
💡Pauli Exclusion Principle
💡Principal Quantum Number (n)
💡Azimuthal Quantum Number (l)
💡Magnetic Quantum Number (m_l)
💡Spin Quantum Number (m_s)
💡Orbitals
💡Electron Configuration
💡Subshells
💡Valence Electron
💡Energy Levels
Highlights
Quantum numbers are essential for determining the location and identity of electrons within an atom.
Each electron in an atom has a unique set of four quantum numbers, ensuring no two electrons share the same address within the atom.
The Pauli Exclusion Principle prohibits two electrons from having identical quantum numbers, especially in the same orbital with the same spin.
Quantum numbers provide an electron's address within the atom, similar to a personal address with a state, city, street, and house number.
The principal quantum number (n) defines the shell or energy level of an electron.
The azimuthal quantum number (l) indicates the subshell type, such as s, p, d, or f.
The magnetic quantum number (m_l) specifies the orientation of an orbital in space, identifying a particular orbital within a subshell.
The spin quantum number (m_s) represents the electron's spin, which can be either up or down.
The range of values for the principal quantum number (n) starts at one and goes to infinity, with no zero or negative values.
The azimuthal quantum number (l) is bounded by the principal quantum number (n), with a maximum value of n-1.
The magnetic quantum number (m_l) takes integer values from -l to +l, with each value corresponding to a different orbital within a subshell.
The spin quantum number (m_s) has only two possible values, +1/2 or -1/2, representing spin up and spin down.
A set of quantum numbers can be used to identify a specific electron in an atom, such as a valence electron in sodium.
For an s subshell, the magnetic quantum number (m_l) is always zero, simplifying the identification of the electron's location.
Understanding quantum numbers is crucial for grasping atomic structure and the behavior of electrons in chemical reactions.
Transcripts
Browse More Related Video

Quantum Numbers, Atomic Orbitals, and Electron Configurations

Quantum Numbers
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers

Lecture 13 - Quantum numbers
Using the Periodic Table to Determine Quantum Numbers - Chemistry Practice Problems
Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle
5.0 / 5 (0 votes)
Thanks for rating: