Energy Levels, Energy Sublevels, Orbitals, & Pauli Exclusion Principle
TLDRThis chemistry lecture delves into the Bohr model of the atom, explaining energy levels, sublevels, and orbitals. Electrons orbit the nucleus in energy levels, which are attracted to the positive charge of the nucleus. The lecture outlines the Pauli exclusion principle, stating that electrons in the same orbital must spin in opposite directions. It also details the number of electrons that can occupy each energy level and sublevel, with a formula for predicting the maximum number of electrons in a shell. The lecture emphasizes the importance of memorizing these concepts for understanding future topics.
Takeaways
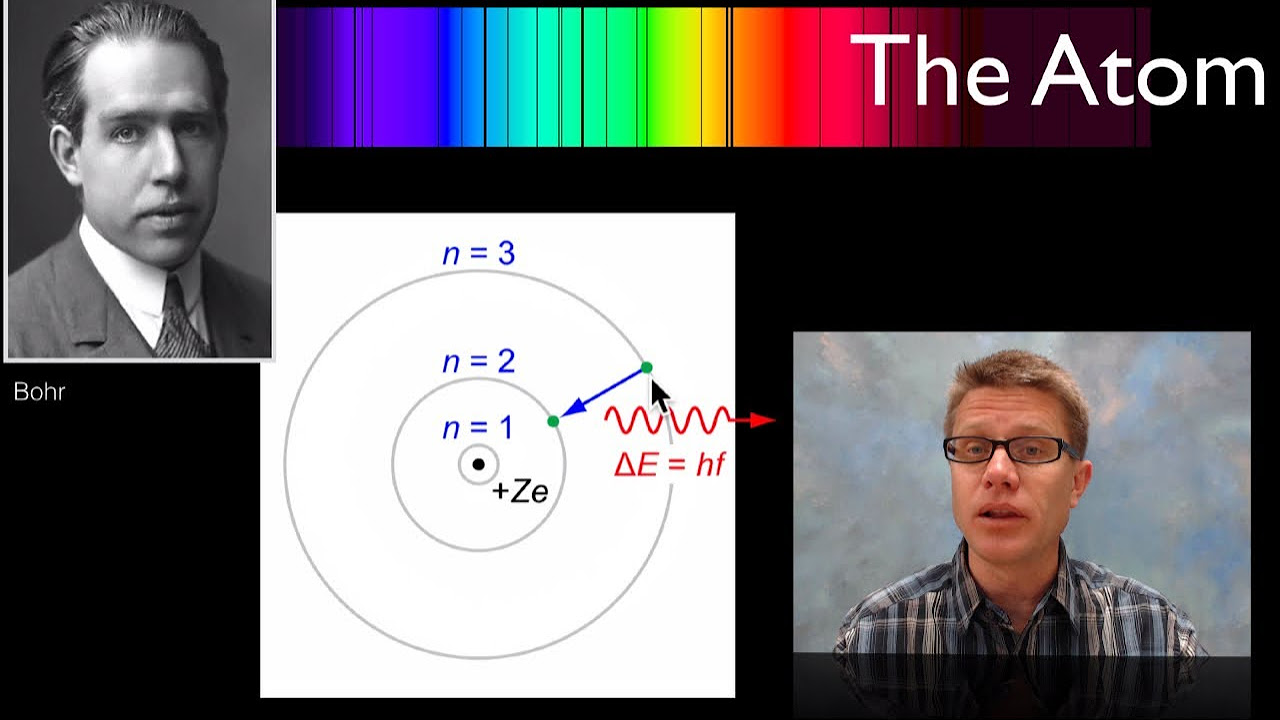
- 🌐 The Bohr model of the atom describes electrons orbiting the nucleus in energy levels, similar to planets around the Sun.
- 🔋 Energy levels are quantized and are further from the nucleus as they increase in number, with higher energy electrons in outermost levels.
- 📈 Principal quantum numbers (n) are used to label energy levels, with lower n values indicating levels closer to the nucleus.
- 🔢 The maximum number of electrons in an energy level follows the formula 2n^2, allowing for the prediction of electron capacity per shell.
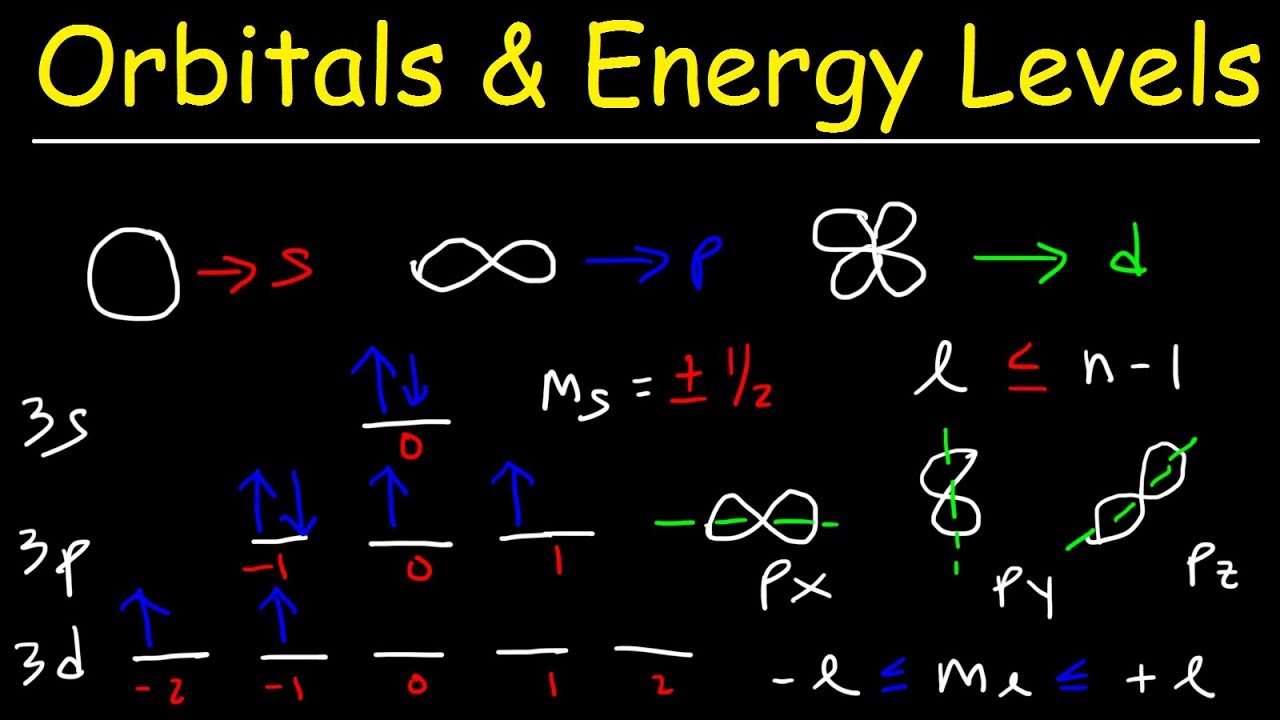
- 🏠 Energy levels contain sublevels labeled as s, p, d, and f, with the number of sublevels equal to the energy level number.
- 🪐 Each sublevel contains orbitals, with s having 1, p having 3, d having 5, and f having 7 orbitals.
- 💫 Electrons within orbitals exhibit spin, and the Pauli exclusion principle states that no two electrons in the same orbital can have the same set of quantum numbers.
- 🔄 Electrons occupying the same orbital must spin in opposite directions, represented by arrows pointing up and down.
- 📊 The structure of the atom can be visualized as a nucleus surrounded by energy levels, sublevels, and finally orbitals where electrons reside.
- 📝 Understanding and memorization of the number of sublevels and orbitals within each energy level is crucial for grasping atomic structure and chemistry concepts.
- 🎓 The lecture emphasizes the importance of memorizing these atomic structure concepts for future learning in chemistry.
Q & A
What is the Bohr model of the atom?
-The Bohr model of the atom is a model where negatively charged electrons orbit around the positively charged nucleus in specific energy levels, similar to how planets orbit the Sun. It was developed by Niels Bohr based on Rutherford's earlier model.
What are energy levels in the context of atomic structure?
-Energy levels are the specific orbits or shells in which electrons reside around the nucleus of an atom. The further an electron is from the nucleus, the higher its energy level.
How are energy levels numbered in atomic structure?
-Energy levels are numbered using principal quantum numbers, denoted by the letter 'n'. The closer an energy level is to the nucleus, the smaller the number.
What is the maximum number of electrons that the first three energy levels can hold?
-The first energy level can hold a maximum of 2 electrons, the second can hold 8, and the third can hold 18 electrons.
What is the formula to predict the maximum number of electrons that can fit into an energy level?
-The formula to predict the maximum number of electrons in an energy level is 2n^2, where 'n' is the principal quantum number of the energy level.
What are sublevels and how are they related to energy levels?
-Sublevels are the divisions within energy levels, and they are labeled as 's', 'p', 'd', and 'f'. Each energy level has a specific number of sublevels, which increases with the energy level number.
How many orbitals does each sublevel type typically have?
-The 's' sublevel has one orbital, 'p' has three, 'd' has five, and 'f' has seven orbitals.
What is the Pauli exclusion principle in the context of electron configuration?
-The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers, which means that an orbital can hold a maximum of two electrons with opposite spins.
How are electrons represented in orbitals according to the Pauli exclusion principle?
-Electrons are represented by arrows pointing up or down to indicate their spin directions. An arrow pointing up represents an electron spinning in one direction, while an arrow pointing down represents an electron spinning in the opposite direction.
What is the significance of understanding the structure of energy levels, sublevels, and orbitals?
-Understanding the structure of energy levels, sublevels, and orbitals is crucial for predicting the behavior of electrons in chemical reactions, understanding atomic spectra, and comprehending the periodic table of elements.
Where can one find a transcript of chemistry lecture number 21?
-A transcript of chemistry lecture number 21 can be found on wwe.com, as mentioned in the closing of the lecture.
Outlines
🔬 Understanding Energy Levels and Sublevels in the Bohr Model
This paragraph introduces the concept of energy levels, sublevels, and orbitals within the context of the Bohr model of the atom. It explains how electrons orbit the nucleus in energy levels, with the outermost levels having higher energy due to the increased effort required to remove an electron from the nucleus. The energy levels are numbered and referred to as principal quantum numbers, denoted by 'n'. The paragraph also discusses the relationship between the energy levels and the number of electrons they can hold, with a formula (2n^2) provided to predict the maximum number of electrons in a shell. Additionally, it touches on the Pauli exclusion principle, stating that no two electrons can occupy the same quantum state simultaneously.
📈 Sublevels and Orbitals: Structure and Capacities
The second paragraph delves deeper into the structure of energy levels, explaining the concept of sublevels (labeled as s, p, d, and f) and their corresponding number of orbitals (1, 3, 5, and 7 respectively). It emphasizes the direct relationship between the number of the energy level and the number of sublevels it contains, likened to an inverted pyramid. The paragraph also describes how each sublevel can hold a specific number of electrons, with an orbital able to accommodate a maximum of two electrons. This section serves as a foundation for understanding the arrangement and distribution of electrons within an atom's structure.
💫 The Pauli Exclusion Principle and Electron Spin
The final paragraph discusses the Pauli exclusion principle in more detail, focusing on how electrons occupy orbitals and their intrinsic property of spinning on their axis. It clarifies that when two electrons share an orbital, they must spin in opposite directions to adhere to the principle. The paragraph introduces the use of arrows to represent spinning electrons, with an upward arrow indicating one direction of spin and a downward arrow indicating the opposite. The summary underscores the importance of memorizing the structure of energy levels, sublevels, and orbitals, as well as the rules governing electron behavior, to fully grasp the subsequent material.
Mindmap
Keywords
💡Energy Levels
💡Sublevels
💡Orbitals
💡Pauli Exclusion Principle
💡Bohr Model of the Atom
💡Rutherford Model of the Atom
💡Electron Configuration
💡Principal Quantum Number (n)
💡Atomic Structure
💡Quantum Numbers
💡Electron Shells
Highlights
Electrons are attracted to the positive nucleus, similar to how planets orbit the Sun.
Rutherford's model of the atom places protons at the center of the nucleus with electrons far away.
Bohr further developed the atomic model by stating that electrons orbit the nucleus.
Energy levels are represented by circles around the nucleus, with outer circles indicating higher electron energy.
The energy required to move an electron away from the nucleus increases with the distance from the nucleus.
Energy levels are numbered starting from 1 and are referred to as principal quantum numbers.
The number of electrons an energy level can hold increases with the energy level number.
The formula to predict the maximum number of electrons in an energy level is 2n^2.
Sublevels within energy levels are labeled as s, p, d, and f, with the number of sublevels equal to the energy level number.
Each sublevel can hold a specific number of orbitals: s has 1, p has 3, d has 5, and f has 7.
Electrons within orbitals must obey the Pauli exclusion principle, which states that no two electrons in the same orbital can have the same set of quantum numbers.
Electrons in an orbital spin on their axis, and if two occupy the same orbital, they must spin in opposite directions.
Arrows are used to represent spinning electrons, with up indicating one direction of spin and down indicating the opposite.
The structure of energy levels, sublevels, and orbitals forms a model of the atom where electrons reside in specific locations based on their energy and spin.
Understanding the structure of energy levels, sublevels, and orbitals is crucial for grasping the concepts in future chemistry lectures.
The lecture provides a detailed visual representation of the atomic structure with electrons in their respective energy levels, sublevels, and orbitals.
The lecture explains the significance of the principal quantum number in determining the energy level of an electron.
The lecture clarifies the relationship between the number of sublevels and the energy level number, which is foundational for understanding atomic structure.
The lecture introduces the concept of orbitals as the final location where electrons reside within the sublevels.
Transcripts
Browse More Related Video
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers

Inside Atoms: Electron Shells and Valence Electron
The Bohr Atom

Electron Configuration

Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy

7.4 Quantum Numbers | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: