Carnot cycle and Carnot engine | Thermodynamics | Physics | Khan Academy
TLDRThe script explores the concept of the Carnot cycle, a foundational principle in thermodynamics, through a thought experiment involving a gas-filled cylinder with a movable piston. It explains how the system interacts with heat reservoirs to maintain constant temperature while expanding or contracting, and how this process results in work being done by the system. The Carnot engine's efficiency is highlighted, emphasizing the theoretical nature of this idealized engine and its role in understanding heat transfer and the concept of entropy.
Takeaways
- 🔬 The script discusses a classic thermodynamics system involving a cylinder with a movable piston and a gas inside, representing an ideal gas scenario.
- 📚 The concept of a reservoir as an infinitely large object with a constant temperature is introduced, used to maintain the temperature of the gas system.
- 🔄 The process of removing weights from the piston to allow the gas to expand while keeping the temperature constant by the presence of a reservoir is explained.
- 📉 The script explains that during the isothermal expansion, the pressure decreases and work is done by the system, but the internal energy remains constant due to constant temperature.
- 📈 The PV diagram is used to illustrate the system's state transitions, showing a move along an isotherm when the temperature is held constant.
- ⚙️ Work done by the system during the isothermal expansion is represented by the area under the curve on the PV diagram.
- 🔥 The script introduces the concept that heat transfer into the system is equal to the work done by the system when the internal energy remains unchanged.
- ❄️ After removing the reservoir, the system is allowed to expand adiabatically, leading to a decrease in pressure, volume, and temperature.
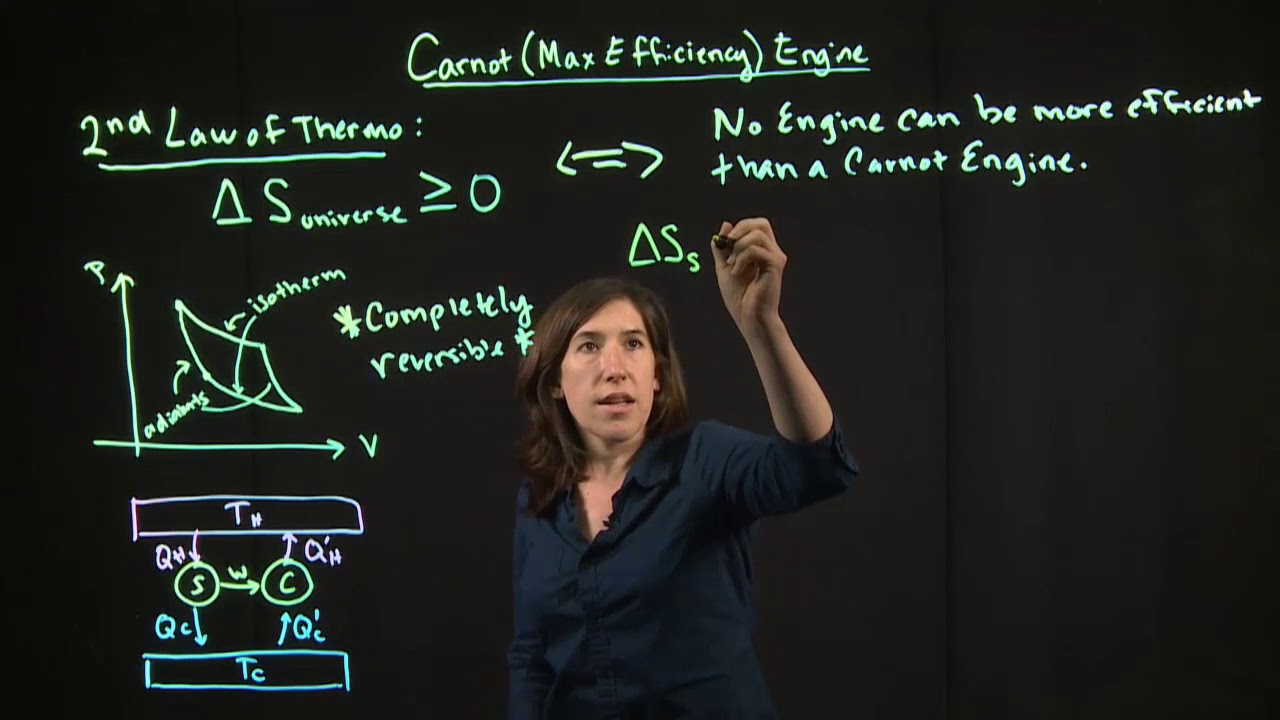
- 🔧 The Carnot cycle is described, which involves four stages including isothermal expansion and compression, and adiabatic expansion and compression, completing a cycle and returning to the initial state.
- 🚀 The Carnot engine is introduced as a theoretical construct to understand the transfer of heat in engines, highlighting that it transfers heat from a hotter to a colder reservoir while doing work.
- 🔑 The script emphasizes the importance of understanding the Carnot cycle for grasping entropy concepts in thermodynamics, noting that heat and work are not state variables.
Q & A
What is the purpose of the movable ceiling in the cylinder described in the script?
-The movable ceiling in the cylinder is there to allow the gas to exert pressure onto it. The movement of the ceiling can be controlled by adding or removing weights (rocks) to simulate changes in pressure and volume of the gas.
What does the term 'adiabatic' mean in the context of the script?
-Adiabatic refers to a process where there is no exchange of heat with the surroundings. In the script, when the pebbles are removed without a reservoir, the system expands and the temperature decreases without any heat transfer, which is an adiabatic process.
What is a reservoir in thermodynamics, as described in the script?
-A reservoir in thermodynamics is a large body of matter or energy that is at a constant temperature. It can be thought of as an infinitely large object that can absorb or supply heat without changing its own temperature significantly.
How does the temperature of the gas in the cylinder remain constant when pebbles are removed, according to the script?
-The temperature of the gas remains constant when pebbles are removed because the system is placed next to a large reservoir that maintains a constant temperature. The reservoir compensates for any changes that would otherwise cause the temperature to decrease.
What is the relationship between pressure, volume, and temperature for an ideal gas at constant temperature?
-For an ideal gas at constant temperature, the product of pressure and volume is constant. This relationship is described by the equation PV = nRT, where n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
What is the significance of the work done by the system in the script?
-The work done by the system is significant as it represents the energy transfer that occurs when the gas expands or is compressed. In the script, the work done by the system is equal to the area under the curve on the PV diagram during the isothermal expansion.
How is the change in internal energy related to heat and work in the script?
-The change in internal energy is equal to the heat added to the system minus the work done by the system, as described by the first law of thermodynamics. In the script, since the temperature remains constant, the internal energy does not change, and the heat added is equal to the work done by the system.
What is an isotherm in the context of the script?
-An isotherm is a curve on a PV diagram that represents all the possible states of a system at a constant temperature. In the script, the system moves along an isotherm when the temperature is kept constant during expansion or compression.
What does the Carnot cycle represent in the script?
-The Carnot cycle represents an idealized thermodynamic cycle that consists of two isothermal processes and two adiabatic processes. It is used to model the operation of a heat engine and is named after Sadi Carnot, who studied the efficiency of heat engines.
What is the efficiency of a Carnot engine, and how is it related to the temperatures of the hot and cold reservoirs?
-The efficiency of a Carnot engine is the ratio of the work done by the engine to the heat absorbed from the hot reservoir. It is determined by the temperatures of the hot and cold reservoirs and is given by the formula 1 - (T2/T1), where T1 is the temperature of the hot reservoir and T2 is the temperature of the cold reservoir.
How does the script explain the concept of entropy in relation to the Carnot cycle?
-The script does not directly explain entropy but implies that understanding the Carnot cycle is essential for grasping the concept of entropy. Entropy is a measure of the thermodynamic disorder within a system, and the Carnot cycle helps to understand how heat transfer is related to entropy changes in thermodynamic processes.
Outlines
🔬 Introduction to the Thermodynamics Experiment
The paragraph introduces a classic thermodynamics experiment involving a movable piston in a cylinder filled with a monoatomic ideal gas. The gas is in equilibrium with a defined volume, pressure, and temperature. The experiment involves placing the system next to a 'reservoir', an infinitely large object with a constant temperature, to maintain the system's temperature as rocks are removed from the top of the piston. This setup is used to explore the behavior of the gas under different conditions, such as when the system is allowed to expand or contract at a constant temperature.
🔧 Work and Heat Transfer in a System
This paragraph delves into the concepts of work done by the system and heat transfer when the system is connected to a reservoir. It explains that the work done by the system is represented by the area under the curve on a PV diagram during an isothermal process. The paragraph also revisits the internal energy formula, stating that the change in internal energy is equal to the heat added to the system minus the work done by the system. Since the internal energy remains constant during the isothermal expansion due to the reservoir's influence, the heat added to the system is equal to the work done by the system.
🌡 Adiabatic Expansion and Isothermal Compression
The third paragraph discusses the process of adiabatic expansion, where the system is isolated and allowed to expand without heat exchange, leading to a decrease in temperature and pressure. Following this, the system is reconnected to a new reservoir at a lower temperature for isothermal compression, which requires the system to release heat to the colder reservoir to maintain the lower temperature. The paragraph emphasizes the importance of understanding the work done and the heat transfer during these processes, as they relate to changes in internal energy.
🔄 The Carnot Cycle and Its Significance
This paragraph introduces the Carnot cycle, a theoretical cycle that represents the most efficient heat engine cycle. It describes the process of returning the system to its original state by adding pebbles (representing pressure) to move from one isotherm to another at a higher temperature, completing the cycle. The Carnot cycle is highlighted as a crucial concept for understanding entropy and the efficiency of heat engines, as it involves transferring heat from a hotter to a colder reservoir while doing work.
🛠 The Carnot Engine and Its Operational Principles
The final paragraph focuses on the Carnot engine, a theoretical construct that operates on the principles of the Carnot cycle. It explains that the engine takes in heat from a hot reservoir, does work, and then transfers the remaining heat to a cold reservoir. The work done by the engine is the difference between the heat absorbed and the heat released. The paragraph concludes by emphasizing the importance of understanding the Carnot cycle and engine for grasping the concepts of entropy and heat transfer in thermodynamics.
Mindmap
Keywords
💡Cylinder
💡Piston
💡Monoatomic Ideal Gas
💡Pressure
💡Reservoir
💡Adiabatic
💡Isothermal Process
💡Quasi-static Process
💡Internal Energy
💡Carnot Cycle
💡Carnot Engine
💡Entropy
Highlights
Introduction to a classic thermodynamics system involving a cylinder with a movable piston and a gas.
Explanation of how rocks offset the force per area of the gas, maintaining equilibrium.
Description of placing the system on top of a reservoir to maintain a constant temperature.
Illustration of how removing pebbles from the system would affect volume, pressure, and temperature in an adiabatic process.
Use of a reservoir to keep the temperature constant as the volume increases.
Introduction of the concept of a quasi-static process where the system is in equilibrium the whole time.
Explanation of how the pressure decreases as the volume increases due to the removal of rocks.
Introduction of the PV diagram and the concept of an isotherm.
Calculation of work done by the system as the area under the curve on the PV diagram.
Discussion on how heat is transferred by the reservoir and its relation to the system's internal energy.
Introduction of the concept of adiabatic process where no heat is exchanged.
Description of the movement from state B to state C in an adiabatic process.
Explanation of how adding pebbles back into the system in an isothermal process affects the temperature.
Introduction of the Carnot cycle and its significance in understanding entropy.
Description of the Carnot engine and its theoretical construct for understanding heat transfer in engines.
Calculation of the work done by the Carnot engine as the difference between heat absorbed and heat released.
Emphasis on the non-state variable nature of heat and work in thermodynamics.
Encouragement for students to understand the Carnot cycle, adiabatic process, and isotherms for a better grasp of entropy.
Transcripts
Browse More Related Video

Carnot efficiency 2: Reversing the cycle | Thermodynamics | Physics | Khan Academy
Carnot Engine
Efficiency of a Carnot engine | Thermodynamics | Physics | Khan Academy

Carnot efficiency 3: Proving that it is the most efficient | Physics | Khan Academy

Work done by isothermic process | Thermodynamics | Physics | Khan Academy

Entropy Change For Melting Ice, Heating Water, Mixtures & Carnot Cycle of Heat Engines - Physics
5.0 / 5 (0 votes)
Thanks for rating: