IR spectra practice | Spectroscopy | Organic chemistry | Khan Academy
TLDRThis video script guides viewers through interpreting IR spectra of different molecules, focusing on the diagnostic region. It explains how to identify carboxylic acids, alcohols, amines, cyclohexane, and ketones by observing specific signals in the double bond region and bond to hydrogen region, highlighting the importance of signal intensity and wave number.
Takeaways
- 🔍 The script discusses the analysis of IR spectra for identifying different molecules based on their characteristic peaks.
- 🧪 The first molecule analyzed is likely an alcohol due to the broad signal in the O-H bond stretch region, ruling out a carboxylic acid and an amine.
- 📉 The absence of a strong carbonyl stretch in the first molecule's spectrum helps eliminate the possibility of it being a carboxylic acid.
- 🔎 The second molecule's IR spectrum shows a signal between 1,600 and 1,700 cm⁻¹, indicating a carbon-carbon double bond stretch, suggesting cyclohexane.
- 🚫 The absence of a triple bond signal in the second molecule's spectrum further supports the identification of cyclohexane.
- 🌐 The third molecule's spectrum has two signals in the double bond region, with one being a strong carbonyl stretch just past 1,700 cm⁻¹, indicative of an unconjugated ketone.
- 🔬 The script emphasizes the importance of the location and intensity of signals in the IR spectrum for identifying molecular structures.
- 📚 The presence of a broad signal in the O-H bond stretch region is a key indicator of hydrogen bonding, which is typical for alcohols.
- 🔍 The script differentiates between conjugated and unconjugated ketones based on the wave number of their carbonyl stretch, with the former having a lower frequency.
- 📈 The script provides a methodical approach to interpreting IR spectra by focusing on the diagnostic regions and comparing expected signal locations and intensities.
- 💡 The script concludes by highlighting the utility of IR spectroscopy in identifying molecular structures through the analysis of specific peaks.
Q & A
What are the three molecules being discussed in the script?
-The three molecules being discussed are a carboxylic acid, an alcohol, and an amine.
What is the significance of the diagnostic region in an IR spectrum?
-The diagnostic region in an IR spectrum is important because it helps in identifying specific functional groups and bonds in a molecule.
Why is the absence of a strong carbonyl stretch in the double bond region indicative of not being a carboxylic acid?
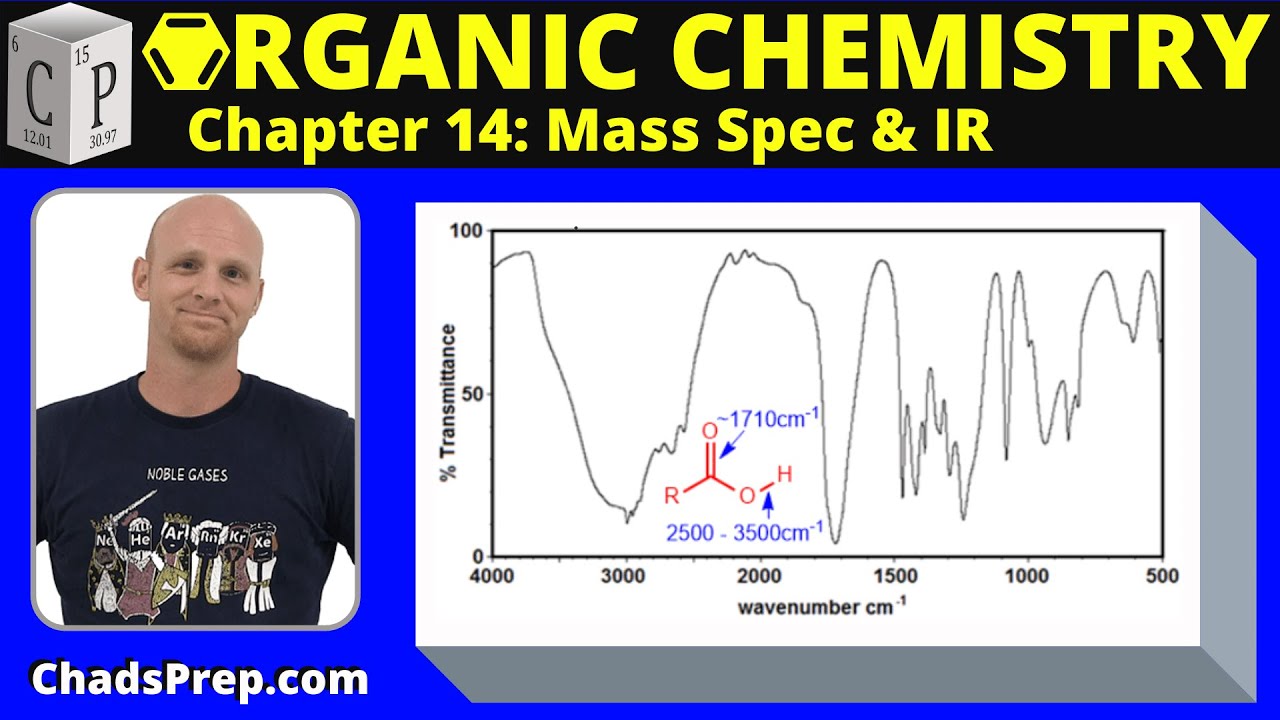
-A carboxylic acid typically has a strong carbonyl stretch just past 1,700 cm⁻¹. If this is absent, it suggests that the molecule is not a carboxylic acid.
What does a broad signal in the bond to hydrogen region suggest?
-A broad signal in the bond to hydrogen region, especially centered on a higher wave number, suggests the presence of hydrogen bonding, typically due to an O-H bond stretch.
How does the presence of a broad O-H bond stretch help in identifying an alcohol in the IR spectrum?
-The broad O-H bond stretch is a characteristic feature of alcohols due to hydrogen bonding. This helps in distinguishing an alcohol from other molecules like carboxylic acids and amines.
What is the typical location and strength of a carbon-carbon double bond stretch in an IR spectrum?
-A carbon-carbon double bond stretch is typically located between 1,600 and 1,700 cm⁻¹ and is not as strong as a carbonyl stretch.
Why can the presence of a signal between 1,600 and 1,700 cm⁻¹ help in identifying a carbon-carbon double bond stretch?
-The signal in this region, though not very strong, is indicative of a carbon-carbon double bond stretch, which helps in ruling out other functional groups like carbonyl or triple bonds.
What is the significance of the absence of a signal in the triple bond region for identifying a molecule?
-The absence of a signal in the triple bond region suggests that the molecule does not contain a triple bond, which can help in narrowing down the possibilities to molecules like cyclohexane.
How does the presence of a signal just higher than 3,000 cm⁻¹ in the bond to hydrogen region help in identifying a molecule?
-A signal just higher than 3,000 cm⁻¹ in the bond to hydrogen region indicates a carbon-hydrogen bond stretch where the carbon is Sp2 hybridized, which is characteristic of molecules like cyclohexane.
What is the difference between the carbonyl stretch of a conjugated ketone and an unconjugated ketone in an IR spectrum?
-The carbonyl stretch of a conjugated ketone is typically lower in wave number than that of an unconjugated ketone due to resonance effects, which decrease the force constant and frequency of vibration.
How does the strength and location of the carbonyl stretch signal help in distinguishing between a conjugated and unconjugated ketone?
-An unconjugated ketone's carbonyl stretch is typically stronger and just past 1,700 cm⁻¹, while a conjugated ketone's carbonyl stretch is weaker and has a lower wave number, often around 1,680 cm⁻¹.
Outlines
🔍 Analyzing IR Spectra for Molecules
In this segment, the focus is on interpreting infrared (IR) spectra to identify specific molecules. The process begins by examining the diagnostic region around 1,500 cm⁻¹, where the absence of a strong carbonyl stretch eliminates the possibility of a carboxylic acid. The presence of a broad signal in the bond to hydrogen region, centered on a higher wave number, indicates hydrogen bonding and suggests an alcohol molecule. The absence of a carbonyl stretch further confirms that the molecule is not a carboxylic acid. Additionally, the lack of two distinct signals for a nitrogen hydrogen bond stretch rules out the amine. The analysis concludes that the IR spectrum corresponds to an alcohol. Further examples involve identifying a cyclohexane molecule by the absence of a triple bond signal and a carbon-hydrogen bond stretch signal at just above 3,000 cm⁻¹. Finally, the presence of two signals in the double bond region, with one just past 1,700 cm⁻¹ and very strong, indicates a carbonyl group, leading to the identification of a conjugated ketone.
🧪 Understanding the Impact of Conjugation on IR Spectra
This paragraph delves deeper into the analysis of IR spectra, specifically focusing on the effects of conjugation on the carbonyl stretch frequency. The discussion starts by identifying a weak signal between 1,600 and 1,700 cm⁻¹, which is indicative of a carbon-carbon double bond stretch. The presence of a strong signal just past 1,700 cm⁻¹ suggests a carbonyl group. The key point here is the distinction between conjugated and unconjugated ketones. In an unconjugated ketone, the carbonyl stretch is expected around 1,715 cm⁻¹, post 1,700 cm⁻¹. However, in a conjugated ketone, resonance effects decrease the force constant, reducing the frequency of vibration, and thus the carbonyl signal is expected to appear at a lower wave number, typically around 1,680 cm⁻¹. The analysis concludes that the observed signal past 1,700 cm⁻¹ corresponds to an unconjugated ketone, providing insight into how to approach the interpretation of simple IR spectra.
Mindmap
Keywords
💡IR spectrum
💡Carboxylic acid
💡Alcohol
💡Amine
💡Carbonyl stretch
💡Bond to hydrogen region
💡Sp3 hybridization
💡Sp2 hybridization
💡Conjugated ketone
💡Unconjugated ketone
Highlights
Introduction to analyzing practice IR spectra for three molecules: carboxylic acid, alcohol, and amine.
Focusing on the diagnostic region around 1,500 for initial analysis.
Elimination of carboxylic acid due to absence of a strong carbonyl stretch.
Identification of broad signal in the bond to hydrogen region as indicative of hydrogen bonding.
Conclusion that the broad signal is due to an O-H bond stretch, suggesting the presence of an alcohol.
Differentiation between carboxylic acid and alcohol based on the absence of a carbonyl stretch in the spectrum.
Elimination of amine due to expected presence of two signals for nitrogen hydrogen bond stretch.
Introduction to a new set of molecules and their IR spectra.
Observation of a weak signal between 1,600 and 1,700, indicative of a carbon-carbon double bond stretch.
Ruling out carbonyl due to the weak signal and its location.
Identification of the molecule as cyclohexane based on the absence of a triple bond signal and the presence of a carbon-hydrogen bond stretch for Sp2 hybridized carbon.
Introduction to a third set of molecules and their IR spectra.
Observation of two signals in the double bond region, suggesting a carbon-carbon double bond stretch and a carbonyl.
Differentiation between conjugated and unconjugated ketones based on the strength and location of the carbonyl signal.
Explanation of how resonance affects the carbonyl signal in conjugated ketones, leading to a lower wave number.
Conclusion that the spectrum corresponds to an unconjugated ketone based on the carbonyl signal being past 1,700 and very strong.
Summary of the approach to analyzing simple IR spectra and identifying molecular structures.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: