How To Balance Chemical Equations
TLDRThis educational video script offers a step-by-step guide on balancing chemical equations, starting with simple examples like chromium and sulfur reactions and advancing to more complex scenarios like combustion reactions involving propane and ethanol. It explains the process of ensuring equal atom counts on both sides of the equation by introducing coefficients and demonstrates the method using examples of mercury oxide decomposition, aluminum and copper chloride reaction, and ammonia with oxygen to form nitrogen monoxide and water. The script emphasizes the importance of balancing elements one by one and provides a clear strategy for tackling challenging equations.
Takeaways
- 🔍 Balancing chemical equations is essential to ensure the number of atoms on both sides of the equation is equal.
- 📐 Start by identifying the least common multiple of atoms to determine the coefficients needed for a balanced equation.
- 🌐 Focus on balancing one element at a time, such as sulfur in the example of chromium and sulfur reacting to form chromium sulfide.
- 🔄 Multiply coefficients to achieve the desired number of atoms for each element, ensuring they match on both sides of the equation.
- 🌀 In the mercury oxide decomposition example, balancing starts with mercury atoms and then adjusts coefficients for oxygen to achieve balance.
- 🛠 For the aluminum and copper chloride reaction, chlorine atoms are balanced first, followed by adjusting the coefficients for aluminum and copper.
- 🔥 In combustion reactions, such as propane with oxygen, start by balancing carbon atoms, then hydrogen, and finally oxygen to complete the balance.
- 💧 When balancing equations involving water (H2O), ensure the number of hydrogen atoms matches by adjusting the coefficient in front of H2O.
- 🔬 For complex reactions, like ethanol combustion, systematically balance carbon, hydrogen, and oxygen atoms to achieve a balanced equation.
- 📉 If you encounter fractions during the balancing process, multiply all coefficients by the denominator to convert them into whole numbers.
- 📝 Remember that coefficients in front of substances represent the number of molecules or atoms involved in the reaction, with '1' being implicit if no number is shown.
Q & A
What is the main focus of the video?
-The main focus of the video is on balancing chemical equations to ensure that the number of atoms on both sides of the equation is equal.
How does the video suggest to start balancing a chemical equation?
-The video suggests starting by focusing on one element at a time, such as sulfur in the example of chromium plus elemental sulfur reacting to form chromium sulfide.
What is the least common multiple method used for in balancing chemical equations?
-The least common multiple method is used to determine the coefficients needed to balance the number of atoms of a particular element on both sides of the equation.
How does the video demonstrate balancing the chromium sulfide example?
-The video demonstrates it by first balancing the sulfur atoms by using the least common multiple of 8 and 3, which is 24, and then balancing the chromium atoms by adjusting the coefficients accordingly.
What is the initial step in balancing the mercury oxide decomposition example?
-The initial step is to ensure that the number of mercury atoms is the same on both sides of the equation, which is already the case in this example.
Why does the video suggest balancing carbon atoms first in combustion reactions?
-Balancing carbon atoms first simplifies the process, as it sets a clear target for the number of carbon dioxide molecules needed on the product side.
How does the video handle the case of fractions when balancing chemical equations?
-If a fraction appears as a coefficient, the video suggests multiplying all coefficients by the denominator of the fraction to eliminate it and achieve whole number coefficients.
What is the purpose of the coefficients in a chemical equation?
-The purpose of the coefficients is to indicate the number of molecules or atoms of each substance involved in the reaction, ensuring the law of conservation of mass is upheld.
How does the video balance the equation for the reaction of aluminum metal with an aqueous solution of copper(II) chloride?
-The video balances it by ensuring the chlorine atoms are equal on both sides by adjusting the coefficients for copper(II) chloride and aluminum chloride, and then balancing the aluminum and copper atoms accordingly.
What is the final step in the video's method of balancing chemical equations?
-The final step is to verify that the number of each type of atom is equal on both sides of the equation, confirming that the equation is balanced.
Outlines
🧪 Balancing Chemical Equations: Basics and Examples
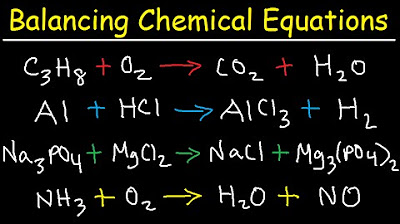
This paragraph introduces the fundamental concept of balancing chemical equations, ensuring equal atom counts on both sides of the equation. The process is illustrated with examples, starting with chromium and sulfur reacting to form chromium sulfide. The method involves identifying reactants and products, then using coefficients to balance sulfur atoms by finding the least common multiple. The chromium atoms are subsequently balanced by adjusting coefficients. Additional examples include mercury oxide decomposing to mercury and oxygen, and aluminum reacting with copper(II) chloride to produce aluminum chloride and copper metal. The paragraph emphasizes the step-by-step approach to balancing equations, focusing on elements one by one.
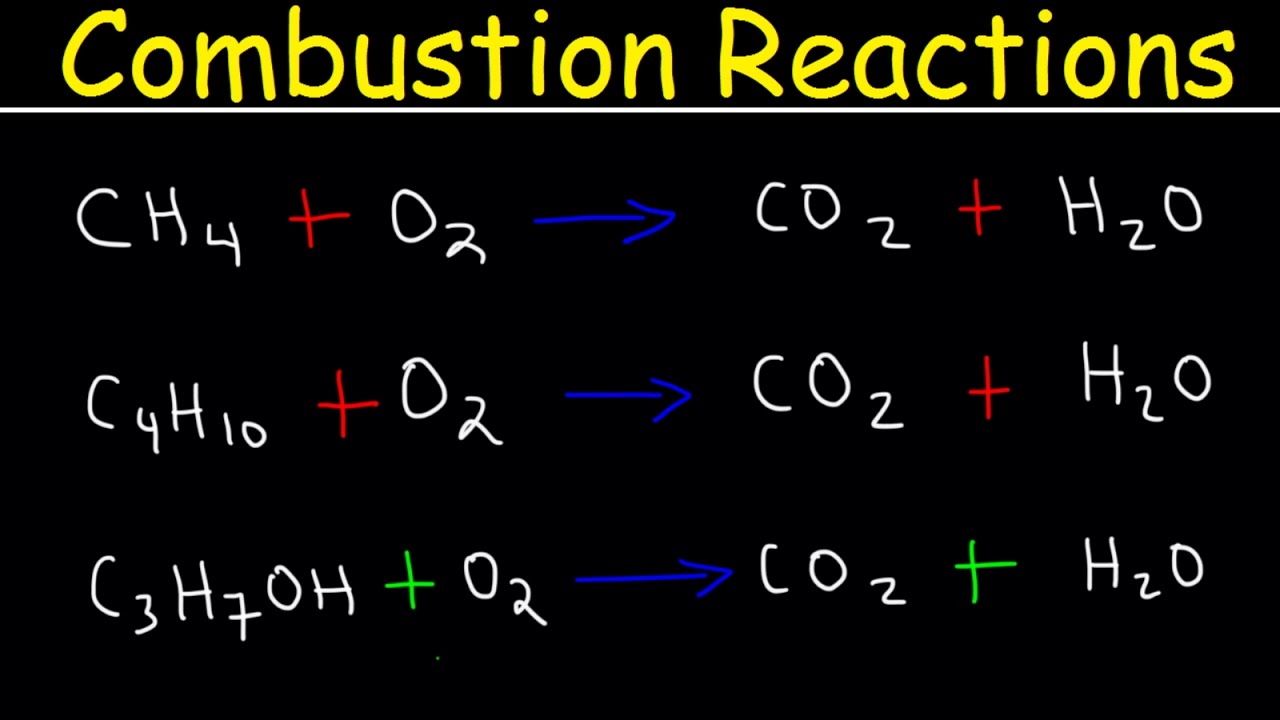
🔥 Advanced Balancing of Combustion Reactions
The second paragraph delves into balancing more complex combustion reactions, starting with propane reacting with oxygen to produce carbon dioxide and water. The summary explains the preference for balancing carbon atoms first, followed by hydrogen, and finally oxygen to ensure the equation is balanced. Another example involves ethanol reacting with oxygen, where the process is similar, focusing on carbon, hydrogen, and then oxygen atoms. A more challenging example of ammonia reacting with oxygen to produce nitrogen monoxide and water is also presented, demonstrating how to deal with fractions by multiplying through to achieve whole number coefficients, thus balancing the hydrogen, nitrogen, and oxygen atoms.
🔄 Resolving Fractional Coefficients in Balancing
The final paragraph continues from the previous example, addressing the challenge of fractional coefficients in chemical equation balancing. It explains the step of multiplying all coefficients by the denominator of the fraction to eliminate it, resulting in whole numbers that balance the equation correctly. The process is demonstrated with the ammonia and oxygen reaction, showing how to adjust coefficients for nitrogen and water molecules to achieve equality on both sides of the equation, thus completing the balance of the reaction.
Mindmap
Keywords
💡Balancing Chemical Equations
💡Reactants
💡Products
💡Coefficients
💡Chromium Sulfide
💡Mercury Oxide
💡Aluminum Chloride
💡Combustion Reaction
💡Ethanol
💡Ammonia
💡Law of Conservation of Mass
Highlights
Balancing chemical equations is essential to ensure an equal number of atoms on both sides of the reaction.
Coefficients are introduced to balance the atoms in a chemical equation.
The least common multiple method can be used to balance elements with small atom counts.
Balancing sulfur atoms in the chromium and sulfur reaction by adjusting coefficients.
Balancing chromium atoms by using the correct coefficients to match sulfur atoms balance.
Mercury oxide decomposes into mercury and oxygen gas when heated.
Balancing mercury and oxygen atoms in the decomposition of mercury oxide.
Aluminum reacts with copper II chloride to form aluminum chloride and copper metal.
Balancing chlorine atoms by adjusting coefficients in the aluminum and copper II chloride reaction.
Balancing copper and aluminum atoms to complete the reaction equation.
Potassium chlorate decomposes into potassium chloride and oxygen gas upon heating.
Balancing oxygen atoms in the decomposition of potassium chlorate.
Propane reacts with oxygen in a combustion reaction to produce carbon dioxide and water.
Balancing carbon, hydrogen, and oxygen atoms in the combustion of propane.
Ethanol reacts with oxygen in another combustion reaction example.
Balancing carbon, hydrogen, and oxygen atoms in the combustion of ethanol.
Ammonia reacts with oxygen to produce nitrogen monoxide and water, presenting a more complex balancing challenge.
Balancing hydrogen and nitrogen atoms in the reaction between ammonia and oxygen.
Dealing with fractions when balancing oxygen atoms by multiplying through by the denominator.
Completing the balance of the ammonia and oxygen reaction by ensuring equal atom counts on both sides.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: