Henry Reaction
TLDRThe Henry reaction, first reported by Louis Henry in 1895, involves the addition of a nitronate salt to a carbonyl compound, usually an aldehyde, and is also known as the nitroaldol reaction. This reaction, important in organic synthesis, produces 1,2-nitroalcohols that can be converted into alpha-hydroxyketones or 1,2-aminoalcohols. The reaction's reversibility and sensitivity to equilibrium pose challenges, while modern advancements, such as those by Masakatsu Shibasaki, have improved stereocontrol using BINOL-metal catalysts. The Henry reaction remains a significant area of research in organic chemistry.
Takeaways
- 🔍 The Henry reaction was discovered by Louis Henry in 1895 and involves the addition of a nitronate salt to a carbonyl compound, typically an aldehyde.
- 📚 Also known as the nitroaldol reaction, it's a significant carbon-carbon bond forming process in organic chemistry.
- ⚗️ Nitronate salts are prepared in situ using strong bases like hydroxide, alkoxide, or amine bases, with a catalytic amount often sufficient to perpetuate the reaction.
- ♻️ The Henry reaction is reversible, with the product able to revert to the starting materials, which can affect the reaction rate and equilibrium.
- 🚫 With ketones, the reaction may be unsatisfactory due to the equilibrium favoring the starting materials, especially because of the weaker acidity of ketones compared to aldehydes.
- ⚠️ Aldehydes used in the reaction are prone to side reactions like aldol condensations and Cannizzaro reactions under basic conditions.
- 🔄 The Henry reaction can be overly favorable, leading to double addition with formaldehyde to form 2-nitro-1,3-diols.
- 🛠 1,2-Nitroalcohols, products of the Henry reaction, are valuable intermediates in organic synthesis, convertible to alpha-hydroxyketones and 1,2-aminoalcohols.
- 🔬 The utility of the nitro group in the Henry product allows for further reactions, such as reduction to amines or dehydration to form nitro-olefins, which are useful in various synthetic pathways.
- 🧬 An example of the Henry reaction's application is the one-carbon homologation of pentoses to hexoses, demonstrating its use in carbohydrate chemistry.
- 💊 The 'double-Henry' reaction has been used in the synthesis of Fingolimod, a therapeutic agent for multiple sclerosis, showcasing the reaction's pharmaceutical relevance.
- 🧬 The Henry reaction can create up to two new stereocenters, making stereoselective synthesis a significant challenge and area of research in organic chemistry.
- 🏆 Professor Masakatsu Shibasaki's work with BINOL-metal catalysts has been pivotal in achieving high levels of stereocontrol in the Henry reaction, particularly with lanthanide derivatives.
Q & A
Who first reported the Henry reaction?
-The Henry reaction was first reported by Belgian chemist Louis Henry in 1895.
What is the general process of the Henry reaction?
-The Henry reaction involves the addition of a nitronate salt to a carbonyl compound, typically an aldehyde, and is sometimes referred to as a nitroaldol reaction.
Why are aliphatic nitro compounds considered carbon acids?
-Aliphatic nitro compounds are considered carbon acids because they have a pKa of around 10, which is as acidic as some 1,3-dicarbonyls like malonates, allowing them to engage in carbanion chemistry.
How is the nitronate typically prepared in the Henry reaction?
-The nitronate is usually prepared in situ by treating the nitroalkane with a strong base such as hydroxide, alkoxide, or even a strong amine base.
Why is the Henry reaction considered reversible?
-The Henry reaction is considered reversible because the product can revert back to the nitronate and aldehyde, and slow reactions are often hindered by unfavorable equilibria.
What are the challenges when using aldehydes in the Henry reaction?
-The use of aldehydes brings the risk of base-promoted reactions like aldol condensations and Cannizzaro reactions, which require careful optimization to avoid.
What is the significance of 1,2-nitroalcohols in organic synthesis?
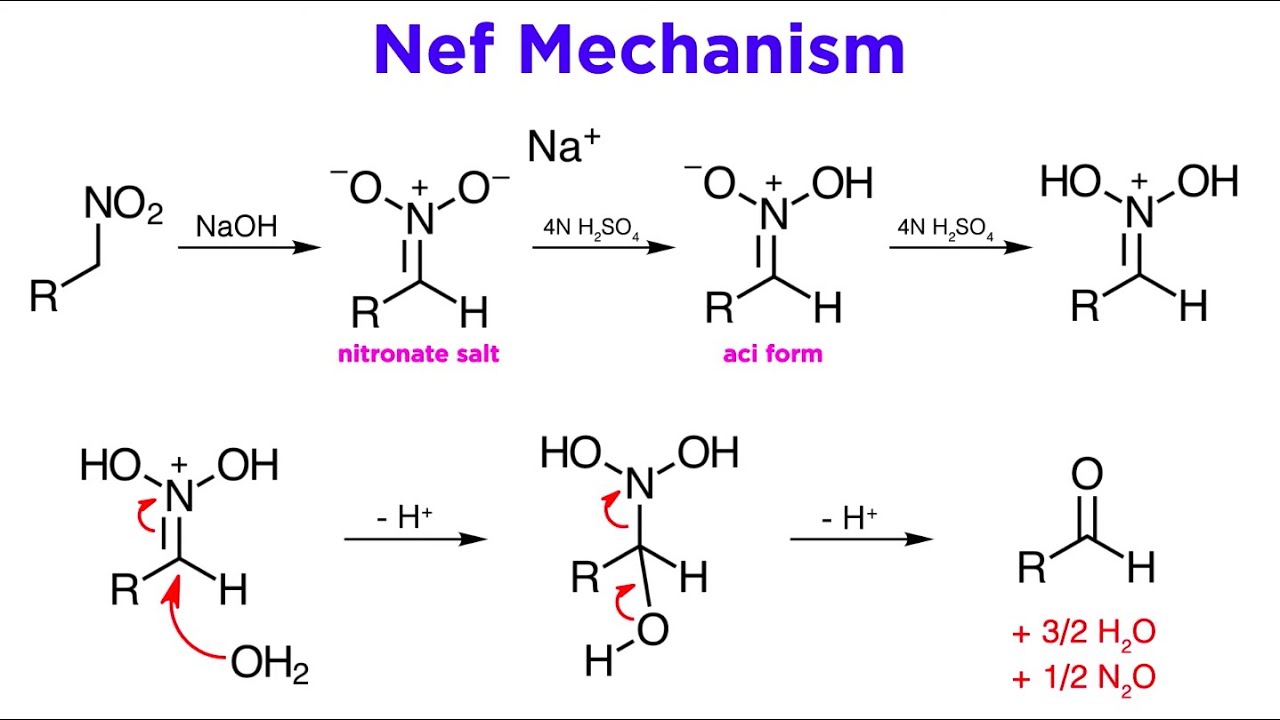
-1,2-Nitroalcohols are important intermediates in organic synthesis, as they can be converted to alpha-hydroxyketones through the Nef reaction and further reduced to 1,2-aminoalcohols.
How can the Henry reaction be used for the one-carbon homologation of pentoses to hexoses?
-The Henry reaction can be used for one-carbon homologation by reacting cyclic sugars in their open chain form with nitromethane, followed by an in situ Nef reaction, resulting in the addition of a carbon atom.
What is the 'double-Henry' reaction and its significance?
-The 'double-Henry' reaction allows for the efficient synthesis of important therapeutic agents, such as Fingolimod for multiple sclerosis, by using two equivalents of formaldehyde and subsequent reduction of the nitro group.
How has the Henry reaction been a target for stereoselective synthesis?
-The Henry reaction has been a prime target for diastereoselective and enantioselective synthesis due to its ability to create up to two new stereocenters, with significant efforts made to achieve excellent stereocontrol.
What is the contribution of Professor Masakatsu Shibasaki to the Henry reaction?
-Professor Masakatsu Shibasaki made a breakthrough by using BINOL-metal catalysts, particularly lanthanide derivatives, to create a chelated, rigid transition state for the Henry reaction, allowing for excellent stereocontrol in the synthesis of compounds like threo-dehydrosphingosine.
Outlines
🔬 Introduction to the Henry Reaction
The Henry reaction, first reported by Louis Henry in 1895, involves adding a nitronate salt to a carbonyl compound, typically an aldehyde. Known also as a nitroaldol reaction, it demonstrates that aliphatic nitro compounds are carbon acids, which can engage in carbanion chemistry. The nitronate is prepared in situ using a strong base, and the reaction is often reversible. Aldehydes used in this reaction can undergo base-promoted side reactions, necessitating careful optimization. Rare cases may see overreaction, forming 2-nitro-1,3-diols. 1,2-Nitroalcohols are key intermediates, convertible to alpha-hydroxyketones, 1,2-aminoalcohols, or nitro-olefins, making the reaction versatile. An example of tandem Henry and Nef reactions is the one-carbon homologation of pentoses to hexoses, where D-arabinose is converted to D-glucose and D-mannose.
📈 Stereoselectivity in the Henry Reaction
The Henry reaction can create up to two new stereocenters, posing a challenge for stereoselective synthesis. Efforts in this area have led to significant advancements, particularly in diastereoselective and enantioselective synthesis. A notable contribution is from Professor Masakatsu Shibasaki, who developed BINOL-metal catalysts for excellent stereocontrol, achieving high selectivity in the synthesis of threo-dehydrosphingosine. This involves lanthanum trichloride organizing three BINOL ligands for a chelated, rigid transition state. Subsequent research has explored other metals and catalytic approaches. The Henry reaction remains a prominent research area in modern organic synthesis and a staple topic in organic chemistry education.
Mindmap
Keywords
💡Henry Reaction
💡Nitronate Salt
💡Carbon Acids
💡Carbanion Chemistry
💡Nef Reaction
💡1,2-Nitroalcohols
💡Stereocenters
💡Diastereoselective and Enantioselective Synthesis
💡BINOL-Metal Catalysts
💡Umpolung
💡Fingolimod
Highlights
The Henry reaction, also known as the nitroaldol reaction, was first reported by Belgian chemist Louis Henry in 1895.
The reaction involves the addition of a nitronate salt to a carbonyl compound, usually an aldehyde.
Nitroalkanes are carbon acids with a pKa of around 10, similar to some 1,3-dicarbonyls, allowing them to engage in carbanion chemistry.
The nitronate is typically prepared in situ by treating the nitroalkane with a strong base, such as hydroxide, alkoxide, or a strong amine base.
The Henry reaction is reversible, and reactions can be hindered by unfavorable equilibria, particularly with ketones.
Aldehydes as reactants in the Henry reaction can lead to base-promoted side reactions like aldol condensations and Cannizzaro reactions.
In rare cases, the Henry reaction can overreact with formaldehyde, forming a 2-nitro-1,3-diol with potential synthetic value.
1,2-Nitroalcohols, important intermediates in organic synthesis, can be converted to alpha-hydroxyketones or 1,2-aminoalcohols.
The Henry reaction product can be dehydrated to a nitro-olefin, a Michael acceptor that can react with various nucleophiles.
The reaction is versatile and extensively used by organic chemists, including in the one-carbon homologation of pentoses to hexoses.
The Henry reaction has been used for the synthesis of Fingolimod, a therapeutic agent against multiple sclerosis, involving a 'double-Henry' reaction.
Stereoselective synthesis in the Henry reaction has been a major focus, with breakthroughs by Professor Masakatsu Shibasaki using BINOL-metal catalysts.
Shibasaki's work demonstrated high stereocontrol and enantioselectivity in the synthesis of threo-dehydrosphingosine using lanthanum trichloride and BINOL ligands.
The Henry reaction continues to be a hot topic of research in modern organic synthesis and is frequently discussed in organic chemistry courses and exams.
Other researchers have extended the work on the Henry reaction to include chiral aldehydes and different catalytic approaches.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: