Predicting Products of Single Replacement Reactions
TLDRThis educational video script explains the process of predicting products in single replacement reactions. It emphasizes the importance of the activity series of metals and halogens to determine if a reaction will proceed. The script illustrates how to identify reactants and products, the concepts of oxidation and reduction, and the solubility rules that affect the final products. It guides viewers through several examples, demonstrating how to balance chemical equations and predict outcomes, such as the formation of metals, gases, and soluble compounds.
Takeaways
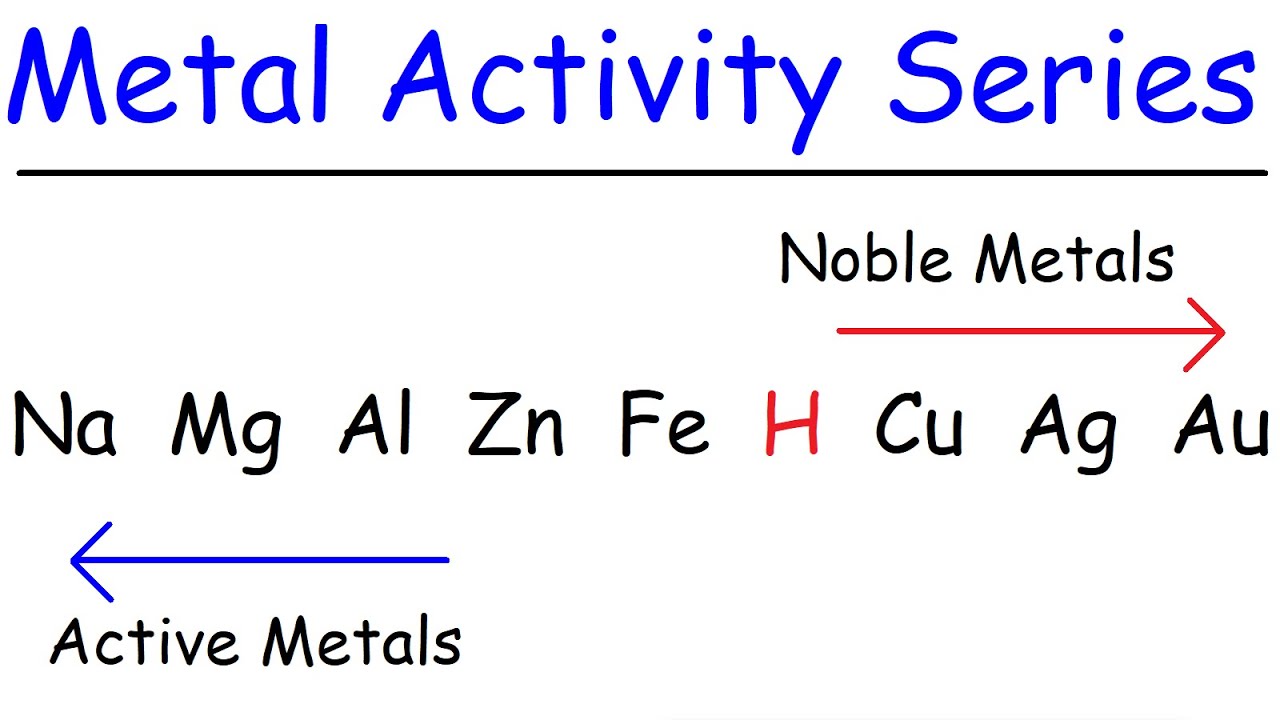
- 🔍 Use the activity series of metals to determine if a single replacement reaction will proceed.
- 📚 Look up the activity series if it's not in your textbook, as it lists metals from most to least reactive.
- 🛑 Zinc can displace copper from a solution because it is more reactive, indicating the reaction will proceed.
- 🔄 In single replacement reactions, a more reactive metal will displace a less reactive metal from its compound.
- 🧪 Predict the products by understanding the changes in oxidation states and the formation of new compounds.
- 🌊 Recognize that nitrates are generally soluble in water, which affects the phase of the products.
- ⚗️ Balance chemical equations by ensuring equal numbers of atoms on both sides for each element.
- 📉 Understand the solubility rules to predict whether a compound will be in the aqueous phase or precipitate.
- 🔋 Oxidation involves a substance giving away electrons, while reduction involves gaining electrons.
- 🌟 The activity series also applies to halogens, which can help predict outcomes in reactions involving non-metals.
- ✅ Practice predicting products and balancing equations to become proficient in understanding single replacement reactions.
Q & A
What is the purpose of the activity series of metals in predicting single replacement reactions?
-The activity series of metals is used to determine if a metal can displace another in a compound, indicating whether a single replacement reaction will proceed. Metals listed earlier in the series are more reactive and can displace those listed later.
How does the position of zinc relative to copper in the activity series affect a potential reaction?
-Since zinc is positioned to the left of copper in the activity series, it is more reactive and can reduce copper ions to copper metal, displacing copper from a solution in a single replacement reaction.
What happens to copper when it is displaced from an aqueous solution by zinc?
-Copper, initially in the form of copper ions in an aqueous solution, will pick up electrons from zinc and precipitate out as copper metal.
What is the chemical formula for zinc nitrate and why is it soluble in water?
-The chemical formula for zinc nitrate is Zn(NO3)2. It is soluble in water because nitrates are generally soluble, and the charges balance out with one zinc ion and two nitrate ions.
Why does the reaction between iron metal and hydrochloric acid proceed?
-The reaction proceeds because iron is positioned to the right of hydrogen in the activity series, meaning it can displace hydrogen from hydrochloric acid to form iron(II) chloride and hydrogen gas.
What is the chemical formula for iron(II) chloride and why is it soluble in water?
-The chemical formula for iron(II) chloride is FeCl2. It is soluble in water because chlorides are generally soluble, except for those of silver, lead, and mercury.
What is the difference between oxidation and reduction in the context of a single replacement reaction?
-Oxidation is the process where a substance gives away electrons, while reduction is the process where a substance acquires electrons. In a single replacement reaction, the metal giving away electrons is being oxidized, and the metal or hydrogen ions acquiring electrons are being reduced.
How does one predict the products of a reaction between aluminum metal and aqueous nickel sulfate?
-Aluminum, being more reactive than nickel, will displace nickel ions to form aluminum sulfate, which is soluble in water, and nickel metal will precipitate out of the solution.
What is the chemical formula for aluminum sulfate and why is it soluble in water?
-The chemical formula for aluminum sulfate is Al2(SO4)3. It is soluble in water because sulfates are generally soluble, except for those of certain group two elements.
What happens when hydrogen gas is bubbled into a solution of copper sulfate?
-Hydrogen, being more active than copper, will be reduced to H+ ions, releasing electrons, which will be picked up by copper ions to form solid copper metal. The H+ ions will combine with sulfate ions to form sulfuric acid, which is soluble in water.
What is the outcome of mixing liquid bromine with a solution of potassium iodide?
-Bromine, being more active than iodine, will displace iodide ions to form elemental iodine, which will precipitate out of the solution, and bromide ions will combine with potassium ions to form soluble potassium bromide.
How does one balance the chemical equation for the reaction between liquid bromine and potassium iodide?
-The chemical equation is balanced by ensuring that the number of atoms of each element on both sides of the equation is equal. For this reaction, placing a 2 in front of KI and KBr achieves balance.
Outlines
🧪 Predicting Products of Single Replacement Reactions
This paragraph introduces the concept of predicting the outcome of single replacement reactions in chemistry. It emphasizes the importance of using the activity series of metals to determine if a reaction will proceed. The video provides a list of metals in order of their reactivity and explains how to use this information to predict whether a metal will displace another in a solution. The example of zinc displacing copper from a solution is used to illustrate the process.
🔬 Balancing Chemical Equations in Single Replacement Reactions
This segment delves into the specifics of balancing chemical equations for single replacement reactions. It explains the process of determining the products of such reactions, including the formation of soluble compounds like zinc nitrate and the precipitation of metals like copper. The paragraph also covers the solubility rules for different compounds and how they affect the outcome of reactions, using iron reacting with hydrochloric acid and aluminum with nickel sulfate as examples.
🌐 Activity Series and Solubility Rules in Halogens Reactions
The final paragraph wraps up the video by discussing the application of the activity series and solubility rules in reactions involving halogens. It uses the example of hydrogen gas being bubbled into a copper sulfate solution, leading to the formation of copper metal and sulfuric acid. The paragraph also covers the reaction between liquid bromine and potassium iodide, resulting in the precipitation of iodine and the formation of potassium bromide, which is soluble in water. The importance of balancing chemical equations is reiterated, and the video concludes with a summary of the key points covered.
Mindmap
Keywords
💡Activity Series of Metals
💡Single Replacement Reaction
💡Oxidation
💡Reduction
💡Polyatomic Ion
💡Solubility Rules
💡Charge Balance
💡Aqueous Phase
💡Precipitate
💡Balancing Chemical Equations
💡Halogens Activity Series
Highlights
Introduction to predicting products of single replacement reactions.
Use of the activity series of metals to determine if a reaction will proceed.
Sodium is more active than magnesium, and magnesium is more active than aluminum, and so on.
Zinc can reduce copper ions to copper metal, demonstrating the use of the activity series.
Explanation of predicting the products of a chemical reaction involving copper and zinc.
Zinc nitrate formation and its solubility in water.
Moving on to the next example involving iron metal and hydrochloric acid.
Iron will pair with chlorine to form FeCl2, demonstrating solubility rules.
Oxidation and reduction processes explained with iron and hydrogen.
Balancing the chemical equation for the iron and hydrochloric acid reaction.
Predicting the products of a reaction between aluminum metal and aqueous nickel sulfate.
Formation of aluminum sulfate and its solubility, with nickel precipitating out.
Balancing the chemical equation for the aluminum and nickel sulfate reaction.
Hydrogen gas reacting with copper sulfate to produce copper metal and sulfuric acid.
Bromine displacing iodide in a solution of potassium iodide, forming elemental iodine and KBr.
Balancing the chemical equation for the bromine and potassium iodide reaction.
Summary of predicting, determining the feasibility, and balancing single replacement reactions.
Transcripts
Browse More Related Video

Predicting Products | Double Replacement Reactions

Chemistry - Will The Reaction Occur?
Activity Series of Metals - Chemistry

Single Replacement Reactions and Net Ionic Equations

Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems

Solving Chemical Reactions - Predicting the Products - CLEAR & SIMPLE CHEMISTRY
5.0 / 5 (0 votes)
Thanks for rating: