Single Replacement Reactions and Net Ionic Equations
TLDRThis educational video script explores single replacement reactions, detailing the process of predicting products and writing balanced equations. It delves into the activity series to determine the feasibility of reactions, explains the crisscross method for formula writing, and distinguishes between total ionic and net ionic equations. The script also highlights the concepts of oxidation and reduction, identifying reducing and oxidizing agents in various reactions involving metals and nonmetals, providing a comprehensive guide to understanding redox chemistry.
Takeaways
- 🔬 Single replacement reactions involve a more reactive metal displacing a less reactive metal in a compound.
- 📚 The net ionic equation is written by identifying and eliminating the spectator ions, which are the same on both sides of the reaction.
- ⚗️ The crisscross method is used to determine the formula between a metal and a non-metal in a compound, by swapping their charges.
- 🧩 Solubility rules are essential in determining the phase (solid, liquid, gas, or aqueous) of the products in a chemical reaction.
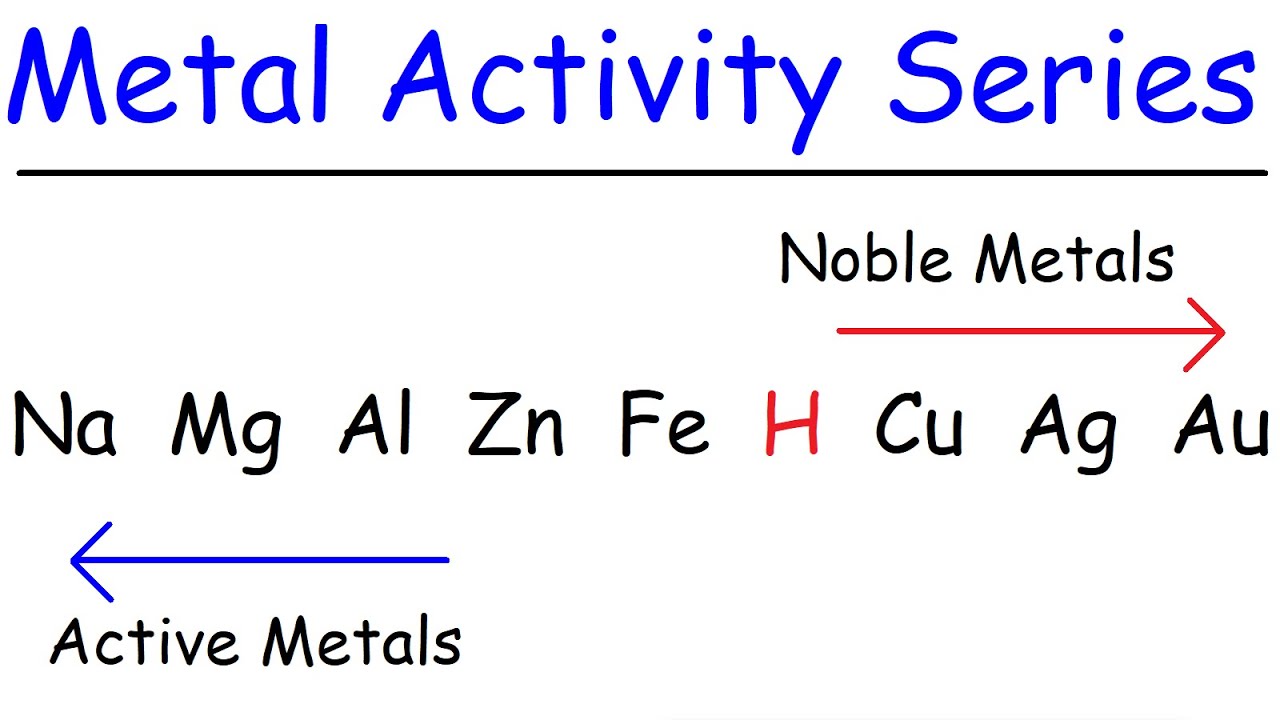
- 📊 The activity series is a guide to predict whether a single replacement reaction will occur, with metals above others in the series being more reactive.
- 🔋 Redox reactions involve the transfer of electrons, where one substance is oxidized (loses electrons) and another is reduced (gains electrons).
- 🛠️ The reducing agent is the substance that gets oxidized, while the oxidizing agent is the one that gets reduced in a reaction.
- 🔄 Balancing chemical equations is crucial to ensure that the number of atoms of each element is the same on both sides of the reaction.
- 🌐 The phase of reactants and products must be considered when writing chemical equations, as it affects the form in which substances participate in reactions.
- 🚫 If a metal is below another in the activity series, it cannot displace the latter from its compound, meaning no reaction will occur.
- 💧 Weak acids like hydrofluoric acid (HF) do not completely ionize in water, so they should not be separated into ions in the ionic equation.
Q & A
What is a single replacement reaction?
-A single replacement reaction, also known as a single displacement reaction, occurs when an element in a compound is replaced by another element from a pure substance.
What happens when aluminum metal is placed in a copper chloride solution?
-In this scenario, aluminum, being more reactive, replaces copper in the copper chloride solution, forming aluminum chloride and solid copper.
How do you write the net ionic equation for a reaction?
-To write the net ionic equation, first write the total ionic equation, separating all the ions in the aqueous phase. Then, eliminate the spectator ions (ions that appear unchanged on both sides of the equation) to reveal the net ionic equation.
What is the crisscross method used for in balancing chemical formulas?
-The crisscross method is used to balance the charges in ionic compounds by swapping the numerical charges of the ions to form the correct chemical formula.
How can you determine if a metal will displace another in a single replacement reaction?
-You can refer to the activity series, which ranks metals by their reactivity. If a metal is higher in the activity series than the metal in the compound, it can displace the latter in a single replacement reaction.
What are the products of the reaction between zinc metal and hydrochloric acid?
-The reaction between zinc metal and hydrochloric acid produces zinc chloride and hydrogen gas.
Why is the reaction between chlorine gas and aqueous sodium bromide feasible?
-The reaction is feasible because chlorine is higher in the activity series for halogens than bromine, allowing it to displace bromide ions to form elemental bromine and sodium chloride.
Why does the reaction between iron metal and zinc chloride not occur?
-The reaction does not occur because iron is lower in the activity series than zinc, meaning it is not reactive enough to displace zinc from zinc chloride.
What are the products of the reaction between sodium metal and hydrochloric acid?
-The reaction between sodium metal and hydrochloric acid produces sodium chloride and hydrogen gas.
How can you identify the oxidizing and reducing agents in a redox reaction?
-The oxidizing agent is the substance that gets reduced (gains electrons), while the reducing agent is the substance that gets oxidized (loses electrons). In the context of the script, aluminum and zinc are reducing agents in their respective reactions, as they lose electrons and are oxidized.
Why is the total ionic equation the same as the net ionic equation for the reaction between sodium and hydrochloric acid?
-In this specific reaction, there are no spectator ions present; all the reactants and products are either in solid, gaseous, or aqueous phase, and the weak acid HF does not fully ionize. Therefore, the total ionic equation directly represents the net ionic equation.
Outlines
🧪 Single Replacement Reactions and Net Ionic Equations
This paragraph introduces single replacement reactions, focusing on the example of aluminum metal in a copper chloride solution. It explains how aluminum, being more reactive, replaces copper to form aluminum chloride and copper metal. The process of writing the balanced chemical equation and net ionic equation is detailed, including the crisscross method for charges and identifying soluble compounds. The activity series is mentioned as a tool to predict the feasibility of such reactions, with aluminum's position above copper indicating the reaction's viability.
🔬 Redox Reactions and Activity Series Application
The second paragraph delves into redox reactions, exemplified by zinc metal reacting with hydrochloric acid. It discusses the activity series' role in determining if zinc can displace hydrogen, which it can. The paragraph outlines the process of balancing the chemical equation, writing the total ionic equation, and then simplifying it to the net ionic equation by removing spectator ions. It highlights the concepts of oxidation and reduction, identifying zinc as the reducing agent and H+ as the oxidizing agent.
🌐 Halogens' Activity Series and Reaction Outcomes
This section examines the reaction between chlorine gas and aqueous sodium bromide, using the activity series of halogens to predict the reaction's outcome. It explains that chlorine, being more reactive, will displace bromine, forming sodium chloride and elemental bromine. The paragraph guides through balancing the chemical equation and writing the total ionic equation, then simplifies it to the net ionic equation, emphasizing the phase of each substance.
⚒ Inactive Metals and Their Reaction Limitations
The fourth paragraph addresses reactions involving metals that are less reactive according to the activity series. Using iron in zinc chloride as an example, it shows that no reaction will occur because iron is below zinc in the series. It also provides an example with sodium and hydrochloric acid, illustrating that sodium, being higher in the series, will react to form sodium chloride and hydrogen gas. The paragraph explains the importance of the activity series in predicting reactions and the process of writing the total ionic equation, noting the difference between strong and weak acids in ionization.
🛠 Final Thoughts on Redox Reactions and Agents
Concluding the video script, the final paragraph wraps up the discussion on redox reactions, using the example of sodium metal with hydrochloric acid. It reiterates the roles of oxidizing and reducing agents, identifying sodium as the reducing agent and HF as the oxidizing agent. The paragraph summarizes the process of writing the total ionic equation for this reaction, noting the absence of spectator ions and the equation's equivalence to the net ionic equation. It ends with a reminder of the phases of each substance involved in the reaction.
Mindmap
Keywords
💡Single Replacement Reaction
💡Net Ionic Equation
💡Activity Series
💡Oxidation
💡Reduction
💡Reducing Agent
💡Oxidizing Agent
💡Spectator Ions
💡Crisscross Method
💡Solubility Rules
💡Phases
Highlights
Introduction to single replacement reactions and the process of identifying products and writing net ionic equations.
Explanation of how aluminum metal can replace copper in a copper chloride solution, forming aluminum chloride and copper metal.
Use of the crisscross method to determine the formula between aluminum and chlorine, resulting in AlCl3.
Understanding solubility rules for chlorides, which are generally soluble except with silver, lead, and mercury.
Balancing the single replacement reaction by finding the least common multiple of chlorine atoms on both sides.
Introduction of the activity series and its importance in determining if a metal can displace another in a solution.
Demonstration of how to write the total ionic equation for the aluminum and copper chloride reaction.
Identification of spectator ions in a reaction and the process of writing the net ionic equation.
Description of single replacement reactions as redox reactions involving electron transfer.
Explanation of the roles of reducing and oxidizing agents in a reaction, with aluminum acting as the reducing agent.
Example of the reaction between zinc metal and hydrochloric acid, including the prediction of products and the reaction's feasibility.
Balancing the reaction between zinc and hydrochloric acid and writing the total ionic equation.
Differentiation between strong and weak acids in terms of their ionization in aqueous solutions.
Analysis of the reaction between chlorine gas and aqueous sodium bromide, including the prediction of products and the reaction's feasibility.
Writing the total and net ionic equations for the chlorine gas and sodium bromide reaction, identifying the oxidizing and reducing agents.
Example of the non-reaction between iron metal and zinc chloride due to iron's position in the activity series.
Final example of the reaction between sodium metal and hydrochloric acid, predicting products, and writing the total ionic equation.
Conclusion summarizing the key points of single replacement reactions, including the roles of reducing and oxidizing agents.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: