Writing Empirical Formula Practice Problems
TLDRThis video tutorial teaches the method of deriving empirical formulas from molecular formulas. It explains the process of simplifying the ratio of atoms in a compound to its simplest form by dividing by the greatest common divisor. Examples provided include C8H18, C8H6O4, C3N12, P2S3, H2O2, and others, illustrating how to reduce numbers to obtain the empirical formula, which may sometimes be identical to the molecular formula if it cannot be further simplified. The video also highlights that different molecular formulas can share the same empirical formula.
Takeaways
- 📚 To write an empirical formula, you simplify the molecular formula to its most reduced form by dividing by the greatest common divisor of all the atoms present.
- 🔍 For C8H18, the empirical formula is derived by dividing the number of carbons and hydrogens by 2, resulting in C4H9.
- 📉 When simplifying, if you can no longer divide the numbers by the same factor, you've reached the simplest form, which is the empirical formula.
- 🌐 For C8H6O4, dividing all the atoms by 2 gives the empirical formula C4H3O2.
- ⚠️ In chemical formulas, a coefficient of 1 is typically not written, so C3N12 simplifies to CN4.
- 🧩 Sometimes, the empirical formula is the same as the molecular formula, as seen with P2S3, which cannot be further reduced.
- 💧 For H2O2, dividing by 2 gives the empirical formula H2O, but since we have a coefficient of 1, it simplifies to just H and O.
- 🔄 Empirical formulas can be the same for different molecular formulas, like C6H6, which has the same empirical formula as several other compounds.
- 🧪 For single-element compounds like S8, the empirical formula is simply the element itself, S, after dividing by 8.
- 🔑 The empirical formula for C6H6 is CH, which is the simplest form of the compound.
- 📉 If a molecular formula cannot be simplified further, as with C12H10, the empirical formula is the same as the molecular formula.
Q & A
What is an empirical formula in chemistry?
-An empirical formula is the simplest whole number ratio of atoms in a compound, representing the compound in its most reduced form.
How do you determine the empirical formula for a compound with the molecular formula C8H18?
-You divide the subscript numbers of all elements by the largest number that can divide both, which in the case of C8H18 is 2, resulting in the empirical formula CH4.
What is the empirical formula for a compound with the molecular formula C8H6O4?
-By dividing all subscript numbers by 2, the empirical formula becomes C4H3O2.
Why do we sometimes not write the number '1' when writing empirical formulas?
-In chemical formulas, the number '1' is typically omitted because it is understood that one atom of an element is present without needing to specify the quantity.
What is the empirical formula for the compound with the molecular formula C3N12?
-Dividing all subscript numbers by 3, the empirical formula is CN4.
Can the empirical formula sometimes be the same as the molecular formula?
-Yes, if the molecular formula is already in its simplest form and cannot be reduced further, it can be the same as the empirical formula, like P2S3.
How do you find the empirical formula for a compound with only one type of element, such as S8?
-Since there's only one type of element and it's already in the form of S8, dividing by 8 gives us S, which is the empirical formula.
What is the empirical formula for the compound C6H6?
-By dividing all subscript numbers by 6, the empirical formula simplifies to CH.
Why might different molecular formulas have the same empirical formula?
-Different molecular formulas can have the same empirical formula because the empirical formula represents the simplest ratio of elements, which can be shared by compounds with different numbers of atoms.
What is the empirical formula for the compound with the molecular formula B3N3H6?
-Dividing all subscript numbers by 3, the empirical formula is BNH2.
What is the process of simplifying a molecular formula to its empirical formula called?
-The process is called reduction, where you divide the subscript numbers by the largest common divisor to get to the simplest form.
Outlines
📚 Writing Empirical Formulas
This paragraph introduces the process of writing empirical formulas for given molecular formulas. The video explains how to simplify the numbers of atoms in a compound to their simplest whole number ratio by dividing by the greatest common divisor. Examples provided include C8H18, which simplifies to CH3, and C8H6O4, which simplifies to CH3O2. The paragraph emphasizes the importance of reducing the formula to its most reduced form and highlights that sometimes the empirical formula can be the same as the molecular formula if it cannot be further reduced.
🔍 Simplifying Molecular to Empirical Formulas
The second paragraph continues the discussion on simplifying molecular formulas to empirical formulas. It covers additional examples such as C3N12, which simplifies to CN4, and P2S3, which remains the same as it cannot be reduced further. The paragraph also touches on the concept that different molecular formulas can share the same empirical formula, such as C6H6, and concludes with the example of H2O2, which simplifies to HO. The summary underscores the method of dividing all elements in a formula by their greatest common divisor to achieve the empirical formula.
Mindmap
Keywords
💡Empirical Formula
💡Molecular Formula
💡Simplification
💡Divisibility
💡Reduced Form
💡Element
💡Compound
💡Fraction
💡Chemical Formula
💡Sulfur (S)
💡Benzene (C6H6)
Highlights
Introduction to writing empirical formulas for molecular formulas.
Example provided: C8H18, a compound with 8 carbons and 18 hydrogens.
Process of simplifying molecular formulas to empirical formulas by dividing by the greatest common divisor.
Demonstration of dividing C8H18 by 2 to get the empirical formula C4H9.
Explanation of when empirical formulas cannot be further reduced.
Example of C8H6O4, showing the division by 2 to simplify the formula.
Clarification on omitting the number '1' in chemical formulas.
Example of C3N12, dividing by 3 to get the empirical formula CN4.
P2S3 example where the molecular formula is already in its simplest form.
H2O2 example, demonstrating division by 2 to get the empirical formula HO.
S8 example, showing that the empirical formula is S when the number is divisible by itself.
C6H6 example, illustrating the division by 6 to get the empirical formula CH.
Discussion on the possibility of multiple compounds having the same empirical formula.
C12H10O example, where the empirical formula is the same as the molecular formula.
B3N3H6 example, dividing by 3 to get the empirical formula BNH2.
Emphasis on the importance of understanding the process of simplifying to empirical formulas.
Encouragement to watch more examples for a deeper understanding of the process.
Transcripts
Browse More Related Video

Empirical Formula and Molecular Formula Introduction

Molecular and Empirical Forumlas from Percent Composition

Calculating Molecular Formula from Empirical Formula

Empirical and Molecular Formulas from Stoichiometry
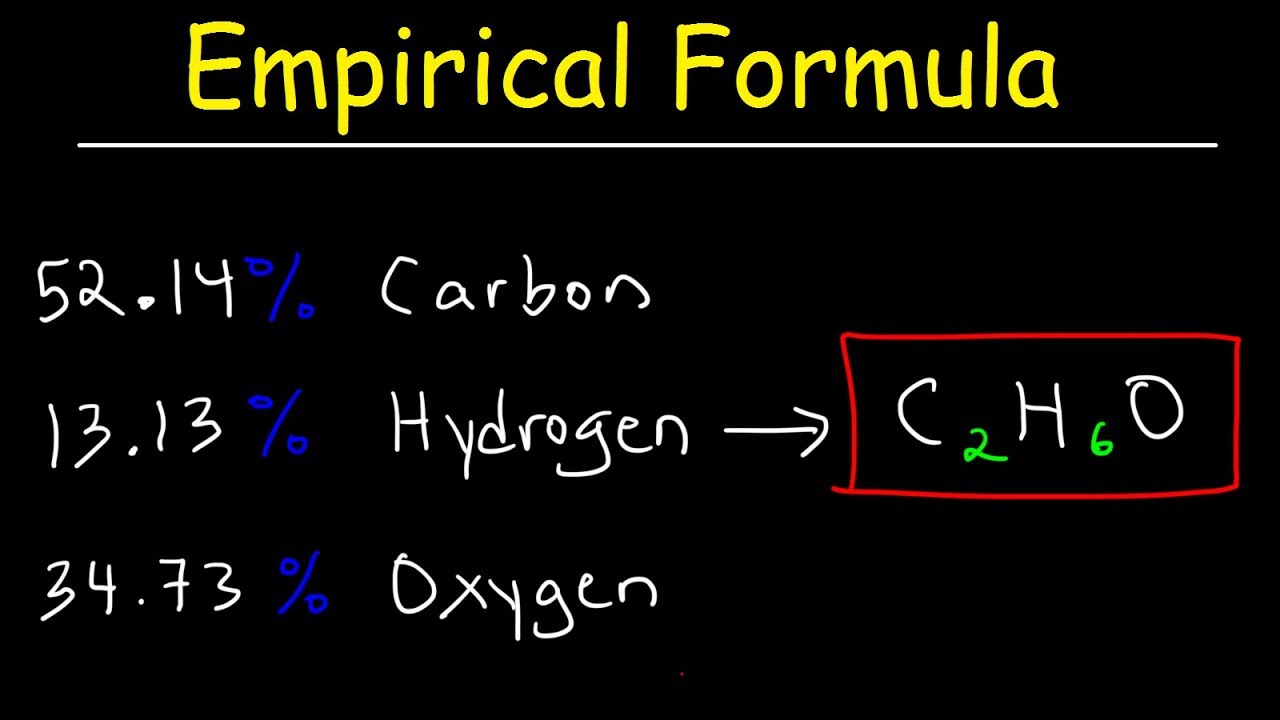
Empirical Formula & Molecular Formula Determination From Percent Composition
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
5.0 / 5 (0 votes)
Thanks for rating: