SN1 Reaction Mechanism
TLDRThis video script delves into the SN1 reaction mechanism, illustrating the process with tert-butyl bromide and potassium iodide. It highlights the formation of carbocation intermediates and the preference of tertiary alkyl halides for SN1 over SN2 reactions. The script explains the unimolecular nature of SN1, contrasting it with bimolecular SN2 reactions. It further explores solvolysis, carbocation rearrangements, and factors affecting the reactivity of different alkyl halides in SN1 reactions, including the influence of steric hindrance and leaving group stability.
Takeaways
- 🧪 The SN1 reaction mechanism involves the substitution of a nucleophile for a leaving group on a substrate, forming a carbocation intermediate.
- 🔄 Tertiary alkyl halides favor the SN1 mechanism over the SN2 due to the stability of the carbocation formed.
- 🏃♂️ The SN1 reaction is a first-order reaction, with the rate depending only on the substrate concentration, not on the nucleophile concentration.
- 🔄 Carbocation rearrangements can occur in SN1 reactions, leading to different products, unlike in SN2 reactions where no such rearrangements happen.
- 🌡 Solvolysis occurs when the solvent acts as a nucleophile, which can lead to the formation of a racemic mixture of products with different stereochemistry.
- 💧 The approach of the nucleophile can be influenced by the presence of the leaving group, affecting the ratio of retention to inversion products.
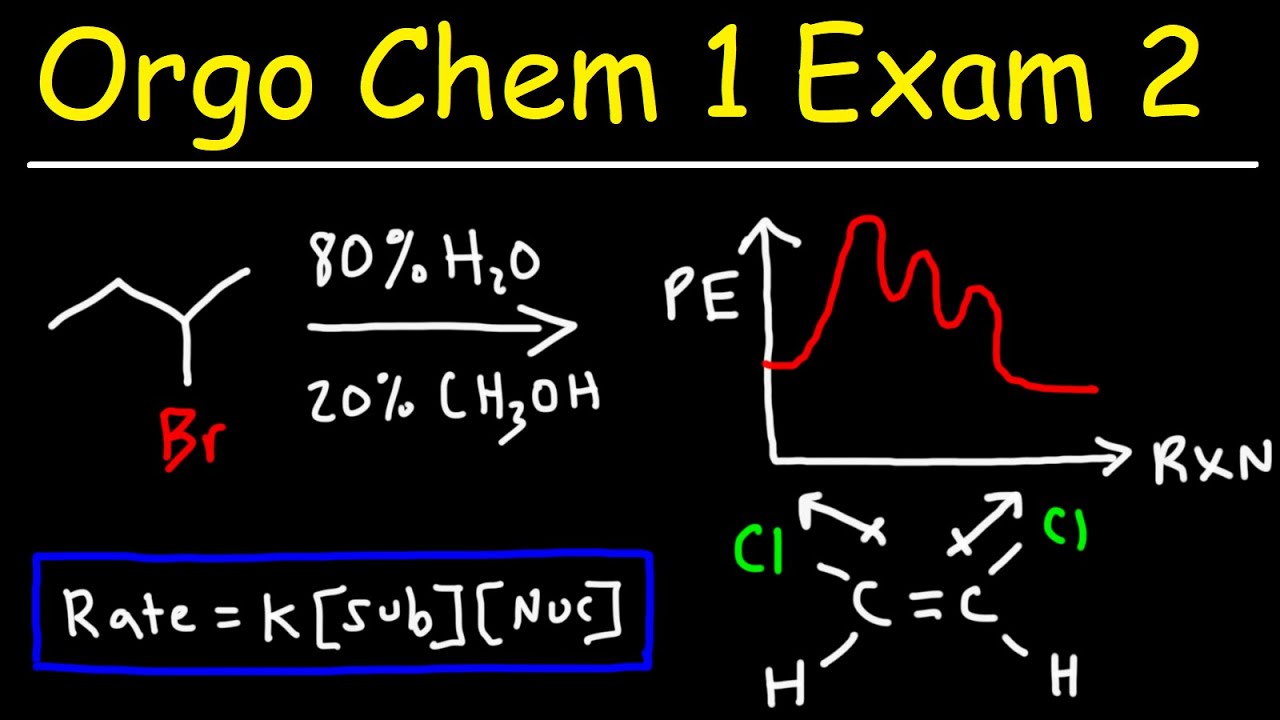
- 📊 The energy diagram of an SN1 reaction typically involves multiple transition states corresponding to the steps of the reaction.
- 🔑 Carbocation stability is crucial for SN1 reactions; tertiary carbocations are more stable due to inductive effects and hyperconjugation.
- 🚫 Primary alkyl halides are generally poor for SN1 reactions due to the instability of primary carbocations, except in cases like allylic systems where resonance can stabilize the carbocation.
- ⛔ Steric hindrance can reduce the yield of SN2 reactions, making SN1 a more favorable pathway for certain substrates, such as neopentyl bromide.
- 📍 The identity of the leaving group affects the reactivity in SN1 reactions, with larger halide ions like iodide being better leaving groups than smaller ones like fluoride.
Q & A
What is the major product when tert-butyl bromide reacts with potassium iodide in an SN1 reaction?
-The major product is tert-butyl iodide, formed by replacing the bromine leaving group with the iodide nucleophile.
Why do tertiary alkyl halides favor the SN1 reaction mechanism over the SN2 mechanism?
-Tertiary alkyl halides favor the SN1 mechanism because they form stable tertiary carbocations, which are essential intermediates in the SN1 process, whereas SN2 reactions are hindered by steric effects.
How does the rate of an SN1 reaction depend on the concentration of the substrate and nucleophile?
-The rate of an SN1 reaction depends only on the concentration of the substrate, not the nucleophile, making it a first-order reaction. Doubling the substrate concentration doubles the reaction rate.
Why do SN1 reactions often produce racemic mixtures of stereoisomers?
-SN1 reactions produce racemic mixtures because the nucleophile can attack the planar carbocation intermediate from either side, leading to both retention and inversion of the original stereochemistry.
What is the difference between the products of retention and inversion in SN1 reactions?
-In SN1 reactions, retention products have the same spatial configuration as the original substrate, while inversion products have the opposite configuration due to attack from the opposite side of the carbocation.
Why do primary alkyl halides generally not undergo SN1 reactions?
-Primary alkyl halides typically do not undergo SN1 reactions because the primary carbocations they form are highly unstable and do not favor the formation of carbocation intermediates required for SN1 mechanisms.
How does a carbocation rearrangement occur in an SN1 reaction?
-Carbocation rearrangement occurs in an SN1 reaction when a less stable carbocation intermediate shifts to a more stable form, typically via hydride or alkyl group shifts, stabilizing the carbocation intermediate.
What role does solvent play in SN1 reactions, and what is solvolysis?
-In SN1 reactions, the solvent can act as both the medium and the nucleophile. Solvolysis is when the solvent itself is the nucleophile, reacting with the carbocation intermediate to form the final product.
Why is iodide a better leaving group than bromide in SN1 reactions?
-Iodide is a better leaving group than bromide because it is larger and can better stabilize the negative charge after leaving, making it more favorable for the bond to break and form a carbocation.
What determines whether an SN1 reaction will occur or not for a given alkyl halide?
-The key factor is the stability of the carbocation intermediate. Alkyl halides that can form stable carbocations, such as tertiary or allylic carbocations, are more likely to undergo SN1 reactions.
Outlines
🧪 SN1 Reaction Mechanism with Tert-Butyl Bromide
This paragraph introduces the SN1 reaction mechanism, focusing on the reaction of tert-butyl bromide with potassium iodide. It explains that the major product is formed by the nucleophilic substitution of the iodide ion for the bromine leaving group. The mechanism involves the ionization of the substrate to form a tertiary carbocation and the subsequent reaction with the nucleophile. The paragraph also contrasts SN1 with SN2 reactions, highlighting that SN1 is a first-order reaction dependent only on substrate concentration, and may involve carbocation rearrangements, unlike SN2.
🌡 Effects of Solvent and Leaving Group on SN1 Reactions
The second paragraph delves into the effects of the solvent and leaving group on the SN1 reaction, using the example of tert-butyl bromide reacting with water. It discusses the concept of solvolysis and the formation of a racemic mixture due to the attack of water from either direction. The paragraph explains the preference for the inverted product due to the repulsion between the negatively charged bromide ion and the partially negatively charged oxygen atom in water, leading to a predominance of the inverted stereoisomer in the product mixture.
🔄 Carbocation Rearrangement in SN1 Reactions
This section discusses carbocation rearrangements that can occur during SN1 reactions, particularly when the reaction involves a secondary alkyl halide adjacent to a tertiary carbon. The mechanism is illustrated with 3-methylbutane, where a hydride shift leads to a more stable tertiary carbocation intermediate. The reaction with water as a nucleophile results in the formation of an ether, and the stereochemistry is considered, noting that the product is not chiral and thus only one SN1 product is formed.
📉 Stability and Reactivity in SN1 Reactions
The fourth paragraph examines the stability and reactivity of different alkyl halides in SN1 reactions. It explains that tertiary alkyl halides are more reactive than secondary or primary ones due to the stabilizing effect of methyl groups on carbocations. The paragraph also touches on exceptions, such as primary allylic alkyl halides, which can undergo SN1 reactions due to the resonance stabilization of the resulting carbocation.
🚫 Limitations of SN1 Reactions with Primary Alkyl Halides
This section addresses the limitations of SN1 reactions with primary alkyl halides, which typically do not form stable carbocations. It contrasts the stability of tertiary carbocations with the instability of primary ones and explains the concepts of inductive effect and hyperconjugation that contribute to carbocation stabilization. The paragraph also notes that certain primary alkyl halides, like allylic ones, can participate in SN1 reactions due to resonance stabilization.
🛑 Steric Hindrance in SN1 and SN2 Reactions
The final paragraph provided discusses the impact of steric hindrance on SN1 and SN2 reactions, using neopentyl bromide as an example. It explains that while primary alkyl halides generally favor SN2 reactions, the presence of a quaternary carbon adjacent to the primary carbon creates steric hindrance that reduces the yield of SN2 reactions. The paragraph concludes by noting that in such cases, an SN1 reaction is more likely to occur, despite the potential for a less favorable yield due to the formation of a tertiary carbocation.
Mindmap
Keywords
💡SN1 Reaction Mechanism
💡Tertiary Alkyl Halides
💡Carbocation
💡Nucleophile
💡Leaving Group
💡Solvolysis
💡Stereoisomers
💡Chiral Carbon
💡Carbocation Rearrangement
💡Allylic Alkyl Halide
💡Steric Hindrance
Highlights
The video focuses on the SN1 reaction mechanism, explaining the major product formation when tert-butyl bromide reacts with potassium iodide.
Tertiary alkyl halides favor the SN1 reaction mechanism over SN2 due to the formation of carbocation intermediates.
SN1 is a first-order nucleophilic substitution reaction, with the rate depending solely on the substrate concentration.
SN2 reactions are second-order reactions, requiring both substrate and nucleophile concentrations for the rate.
The mechanism of solvolysis is discussed, where water acts as both a solvent and nucleophile in the reaction.
Two different products result from the solvolysis of tertiary alkyl halides with water, leading to a racemic mixture.
The retention and inverted products from solvolysis are distinguished by the approach of the nucleophile to the carbocation.
The bromide ion's presence influences the formation of retention and inverted products due to electrostatic repulsion.
An energy diagram for the SN1 reaction is sketched, illustrating the transition states for a three-step process.
Carbocation rearrangements are highlighted as a key aspect of the SN1 mechanism, especially with secondary alkyl halides adjacent to tertiary carbons.
The stability of carbocations is explained through inductive effect and hyperconjugation, favoring tertiary over primary carbocations.
Allylic alkyl halides are exceptions to the general reactivity trend in SN1 reactions due to resonance stabilization.
Primary alkyl halides typically do not undergo SN1 reactions due to the instability of primary carbocations.
Neopentyl bromide is shown to undergo an SN1 reaction despite being a primary alkyl halide, due to a unique rearrangement.
The impact of steric hindrance on SN2 reactions is discussed, particularly with quaternary carbons adjacent to primary alkyl halides.
A comparison of alkyl halides' reactivity in SN1 reactions is provided, with tertiary alkyl halides being the most reactive.
The video concludes with a series of questions to test the viewer's understanding of SN1 reaction mechanisms and substrate reactivity.
Transcripts
Browse More Related Video

SN1 SN2 E1 E2 Reaction Mechanism - Test Review

SN2 Reaction Mechanisms

Practice drawing SN1 vs SN2 reaction mechanisms and products with more than 9 examples

Nucleophilic Substitution Reactions - SN1 and SN2 Mechanism, Organic Chemistry

7.3 SN1 vs SN2 | Organic Chemistry
Organic Chemistry 1 Exam 2 Review
5.0 / 5 (0 votes)
Thanks for rating: