Molarity, molality, osmolarity, osmolality, and tonicity - what's the difference? | Khan Academy
TLDRThis educational script clarifies the distinctions between molarity, molality, osmolarity, osmolality, and tonicity—key concepts in chemistry and medicine. Molarity and molality are differentiated by their denominators, with the former referring to moles per liter of solution and the latter to moles per kilogram of solvent. Osmolarity and osmolality also share a similar structure, differing in their denominators: liters of solution for the former and kilograms of solvent for the latter. Tonicity, which includes hypotonic, isosmotic, and hypertonic solutions, describes the relationship between two solutions separated by a membrane, commonly used in medical contexts to discuss the balance of fluids inside and outside cells.
Takeaways
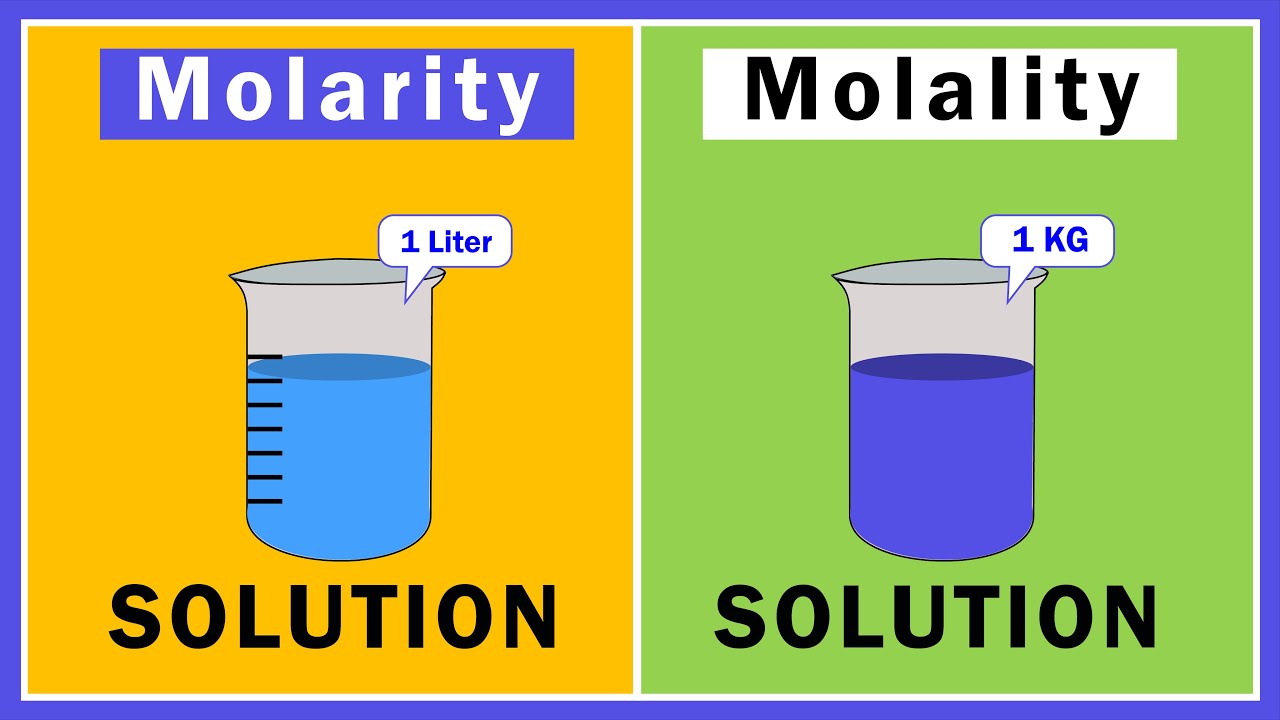
- 🧪 The term 'molarity' refers to moles of solute per liter of solution.
- 📏 'Molality' is similar to molarity but uses kilograms of solvent instead of liters of solution, with moles of solute in the numerator.
- 🌊 'Osmolarity' retains the one liter of solution denominator but changes the numerator to osmoles, which accounts for the number of particles a solute can dissociate into.
- 🍯 'Osmolality' is like osmolarity but uses one kilogram of solvent in the denominator, with osmoles in the numerator.
- 🔄 The major difference between molarity/molality and osmolarity/osmolality is the denominator, which refers to the volume or mass of the solution or solvent.
- 💉 'Tonicity' is a term used to describe the relative concentration of solutes in two solutions separated by a membrane, categorized as hypotonic, isontonic, or hypertonic.
- 🔬 Tonicity is particularly relevant in medical contexts, where it can affect the movement of water across cell membranes.
- 🌡 The terms molarity, molality, osmolarity, and osmolality are used to describe a single solution's concentration.
- 🔄 Tonicity, on the other hand, is used to compare two solutions separated by a semipermeable membrane.
- 🚑 These terms are crucial in medicine, especially when considering the effects on cells and their permeable membranes in various biological fluids like blood or interstitial fluid.
- 📚 Understanding the differences between these terms helps in accurately describing and comparing solutions in medical and scientific contexts.
Q & A
What does the term 'molarity' refer to in the context of solutions?
-Molarity refers to the number of moles of solute in one liter of solution.
How does the definition of 'molality' differ from 'molarity'?
-Molality is defined as the number of moles of solute in one kilogram of solvent, rather than one liter of solution as in molarity.
What is the main difference between 'molarity' and 'molality' in terms of their denominators?
-The main difference lies in the denominator: molarity uses one liter of solution, while molality uses one kilogram of solvent.
What does 'osmolarity' measure in a solution?
-Osmolarity measures the number of osmoles in one liter of solution, taking into account the potential for substances to dissociate.
How is 'osmolality' related to 'osmolarity'?
-Osmolality has the same numerator as osmolarity, which is osmoles, but the denominator is one kilogram of solvent instead of one liter of solution.
What is the key difference between 'osmolarity' and 'osmolality'?
-The key difference is the denominator: osmolarity uses one liter of solution, while osmolality uses one kilogram of solvent.
What does 'tonicity' describe in the context of solutions?
-Tonicity describes the relative concentration of solutes in solutions separated by a membrane, categorized as hypotonic, isotonic, or hypertonic.
How do the terms 'hypotonic', 'isotonic', and 'hypertonic' relate to 'tonicity'?
-These terms are classifications of tonicity, indicating whether a solution has a lower, equal, or higher concentration of solutes compared to another solution separated by a membrane.
What is the primary application of these terms (molarity, molality, osmolarity, osmolality, tonicity)?
-These terms are primarily used in medicine to describe the concentration of solutions and their effects on cells with permeable membranes.
Why are these terms important in the context of cells and permeable membranes?
-They are important because they help to describe the relationship between the solution inside and outside of cells, which can affect cell function and integrity.
Can you provide an example of how 'salt' differs in molarity and osmolarity?
-Salt, when dissolved, can dissociate into ions. This means that its molarity (moles of salt) may not reflect the total osmotic effect, hence its osmolarity (moles of osmoles) can be different.
Outlines
🧪 Chemistry of Solutions: Molarity, Molality, Osmolarity, and Osmolality
The video script begins by clarifying the differences between several terms used in chemistry that describe solutions. It starts with 'molarity,' which is defined as moles of solute per liter of solution. The script then introduces 'molality,' which is similar to molarity but uses kilograms of solvent instead of liters of solution. Next, 'osmolarity' is explained, keeping the volume of solution constant but changing the numerator to osmoles, which accounts for the number of particles that can result from the dissociation of solute molecules. 'Osmolality' is similar to osmolarity but uses kilograms of solvent in the denominator. The script also touches on 'tonicity,' which is related to the relative concentration of solutes in solutions separated by a membrane, and includes hypotonic, isotonic, and hypertonic solutions. The explanation aims to help viewers understand how these terms are used in medical contexts, particularly in relation to cells and permeable membranes, such as those found in blood and interstitial fluid.
Mindmap
Keywords
💡Molarity
💡Molality
💡Osmolarity
💡Osmolality
💡Tonicity
💡Hypotonic
💡Isosmotic
💡Hypertonic
💡Permeable Membrane
💡Interstitial Fluid
💡Medical Terms
Highlights
The main difference between molarity and molality is the denominator: molarity uses liters of solution, while molality uses kilograms of solvent.
Molarity is defined as moles in one liter of solution.
Molality is defined as moles in one kilogram of solvent.
Osmolarity is similar to molarity but uses osmoles instead of moles.
Osmoles consider how substances can split apart in a solution, such as salt.
Osmolarity is measured in osmoles per liter of solution.
Osmolality is similar to osmolarity but uses kilograms of solvent in the denominator.
Osmolality is measured in osmoles per kilogram of solvent.
Tonicity describes the relative concentration of solutions separated by a membrane.
Tonicity can be categorized into hypotonic, isosmotic, and hypertonic solutions.
The terms molarity, molality, osmolarity, and osmolality are used to describe a single solution.
Tonicity is used to describe the relationship between two solutions separated by a membrane.
These terms are particularly relevant in medical contexts, such as the concentration of solutions around cells.
Cells with permeable membranes are a key element in understanding the application of these terms in medicine.
The solution surrounding a cell, such as blood or interstitial fluid, is crucial for understanding tonicity.
The relationship between the solution inside and outside of cells is central to the discussion of these terms.
These terms help to quantify and compare the concentration of solutes in different solutions.
Transcripts
Browse More Related Video

Molarity versus Molality
What is molarity and molality Class 11? | What is molality and example? | calculate molality

Difference between Molarity and Molality

What's the Difference Between Molarity and Molality?

Molarity vs. molality | Lab values and concentrations | Health & Medicine | Khan Academy

What's the Point of Molality?!?
5.0 / 5 (0 votes)
Thanks for rating: