Difference between Molarity and Molality
TLDRThis educational script introduces the concepts of molarity and molality, two measures used to determine the concentration of a solution. Molarity is defined as the number of moles of solute dissolved in one liter of solution, disregarding the solvent, and is denoted by 'M'. Molality, on the other hand, is the number of moles of solute per 1 kg of solvent. The script provides examples of how to prepare solutions with specific molarities and molalities, emphasizing the importance of understanding these terms for accurate measurement and preparation of solutions. It promises to teach easy tricks for calculating molarity and molality in a subsequent lecture.
Takeaways
- 🧪 Concentration is defined as the amount of solute dissolved in a given amount of solvent.
- 📚 Molarity and molality are terms used to measure the concentration of a solution.
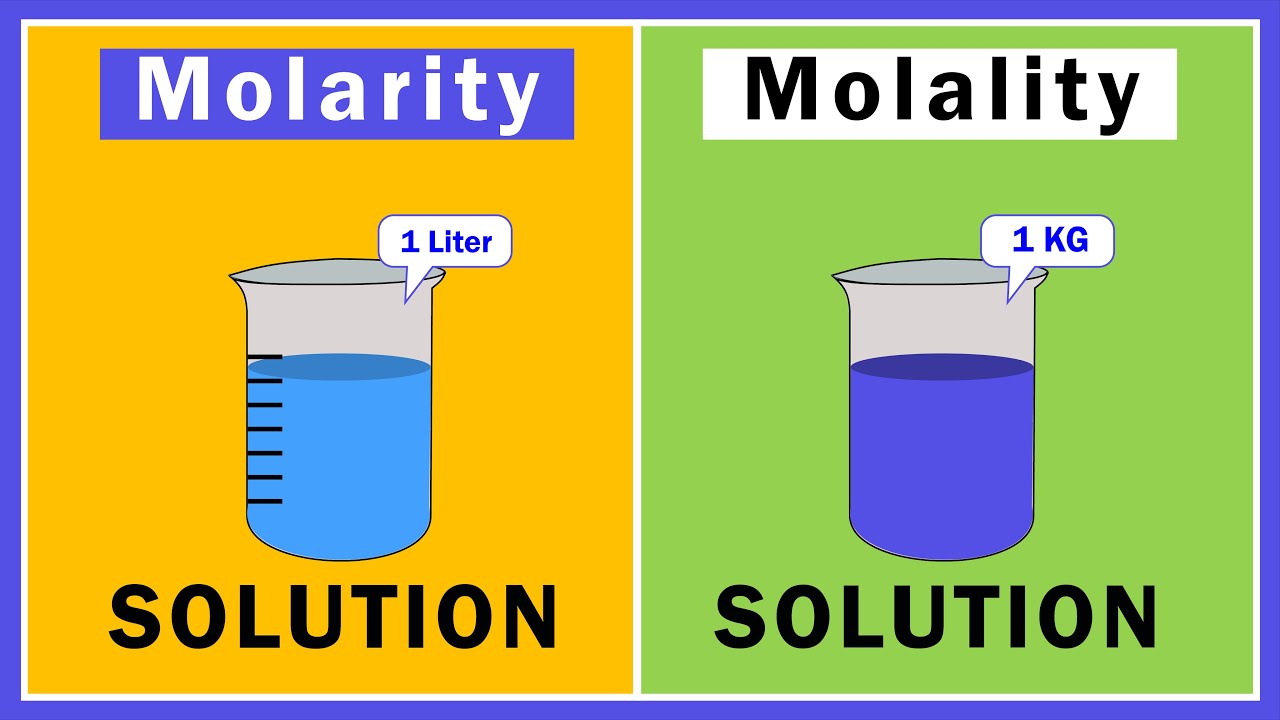
- 📏 Molarity measures the number of moles of solute per liter of solution, ignoring the solvent.
- 🔍 Molarity is denoted by 'M' and is calculated as the number of moles of solute divided by the volume of the solution in liters.
- 🧪 To prepare a solution with a specific molarity, dissolve the required number of moles of solute in water until the total volume reaches one liter.
- 📏 Molality measures the number of moles of solute per kilogram of solvent.
- 🔍 Molality is denoted by 'm' and is calculated as the number of moles of solute divided by the mass of the solvent in kilograms.
- 🧪 To prepare a solution with a specific molality, dissolve the required number of moles of solute in one kilogram of solvent.
- 📚 Understanding molarity and molality is crucial for accurately measuring and comparing the concentrations of different solutions.
- 🔑 The script promises to teach an easy trick to calculate molarity and molality in the next lecture.
Q & A
What is the basic concept of concentration in chemistry?
-Concentration is defined as the amount of solute dissolved in a given amount of solvent. It helps in understanding how much of a substance is present in a solution.
How does the concentration of a solution relate to the amount of solute dissolved?
-The concentration of a solution is directly related to the amount of solute dissolved. More solute results in a more concentrated solution, while less solute results in a less concentrated solution.
What are the two common terms used to measure the concentration of a solution?
-The two common terms used to measure the concentration of a solution are molarity and molality.
What does molarity measure in terms of a solution?
-Molarity measures the number of moles of solute dissolved in one liter of solution, not considering the volume of the solvent.
How is molarity denoted and what is its formula?
-Molarity is denoted by the capital letter 'M' and its formula is the number of moles of solute divided by the volume of the solution in liters.
Can you provide an example of how to prepare a solution with a specific molarity?
-To prepare a solution with a specific molarity, such as 5M HCl, you would take 5 moles of HCl and add water slowly until the total volume of the solution is one liter.
What is the difference between molarity and molality?
-Molarity measures the moles of solute per liter of solution, while molality measures the moles of solute per kilogram of solvent.
How is molality denoted and what is its formula?
-Molality is denoted by the lowercase letter 'm' and its formula is the number of moles of solute divided by the mass of the solvent in kilograms.
Can you give an example of preparing a solution with a specific molality?
-To prepare a solution with a specific molality, such as 5m HCl, you would take 1 kg of water and add 5 moles of HCl to it.
What is the significance of the 'm' notation when it appears with a number before it?
-The 'm' notation followed by a number indicates molarity (e.g., 5M means 5 moles per liter) or molality (e.g., 5m means 5 moles solute dissolved in 1 kg solvent).
What will be covered in the next lecture regarding molarity and molality?
-The next lecture will teach an easy trick to calculate molarity and molality.
Outlines
🧪 Understanding Molarity and Molality
This paragraph introduces the concepts of molarity and molality, two measures of solution concentration. It explains that concentration refers to the amount of solute dissolved in a given amount of solvent, using the example of sugar dissolved in water. The paragraph then distinguishes between molarity, which is the number of moles of solute per liter of solution (not considering the solvent), and molality, which is the number of moles of solute per kilogram of solvent. It provides the formulas for both and gives examples of how to prepare solutions with specific molarities and molalities of HCl. The paragraph concludes by noting that the next lecture will cover easy tricks for calculating these concentrations.
Mindmap
Keywords
💡Concentration
💡Molarity
💡Molality
💡Solute
💡Solvent
💡Solution
💡Mole
💡Beaker
💡Liter
💡Kilogram
Highlights
Concentration is defined as the amount of solute dissolved in a given amount of solvent.
Molarity and molality are terms used to measure the concentration of a solution.
Molarity measures the number of moles of solute dissolved in one liter of solution.
The solvent's volume is not considered in molarity calculations.
Molarity is denoted by 'M' and calculated as moles of solute over the volume of the solution.
Example given: Preparing a 5M HCl solution by dissolving 5 moles of HCl in one liter of water.
Molarity is expressed in moles per liter, e.g., 5M means 5 moles per liter.
Molality measures the number of moles of solute dissolved in 1 kg of solvent.
In molality, the mass of the solvent is considered in the calculation.
Molality is denoted by 'm' and calculated as moles of solute over the mass of the solvent.
Example given: Preparing a 5m molality HCl solution by adding 5 moles of HCl to 1 kg of water.
Molality is expressed in moles per kilogram of solvent, e.g., 5m means 5 moles per 1 kg of solvent.
Molarity is volume-dependent, whereas molality is mass-dependent.
Understanding the difference between molarity and molality is crucial for accurate solution preparation.
An easy trick to calculate molarity and molality will be taught in the next lecture.
The importance of remembering the formulas and units for molarity and molality.
The transcript provides a clear distinction between molarity and molality for better understanding.
Transcripts
Browse More Related Video
What is molarity and molality Class 11? | What is molality and example? | calculate molality

Molarity vs. molality | Lab values and concentrations | Health & Medicine | Khan Academy

Molarity versus Molality

What's the Difference Between Molarity and Molality?

Concentration and Molarity explained: what is it, how is it used + practice problems

How to Calculate Molality
5.0 / 5 (0 votes)
Thanks for rating: