What's the Difference Between Molarity and Molality?
TLDRThis script explores the concepts of molarity and molality, two measures of concentration that may sound similar but have distinct differences. Molarity is defined as the moles of solute per liter of solution, considering both solute and solvent. In contrast, molality is the moles of solute per kilogram of solvent, focusing solely on the solvent's mass. The script clarifies these differences through the example of making a molar and molal solution of calcium chloride. It also highlights the practical advantage of molality, which simplifies the preparation process by using a scale instead of measuring volumes. The script invites viewers to learn more about the technical differences in a follow-up video.
Takeaways
- 😀 Marity and molality are both measures of concentration, representing the amount of solute in a solution.
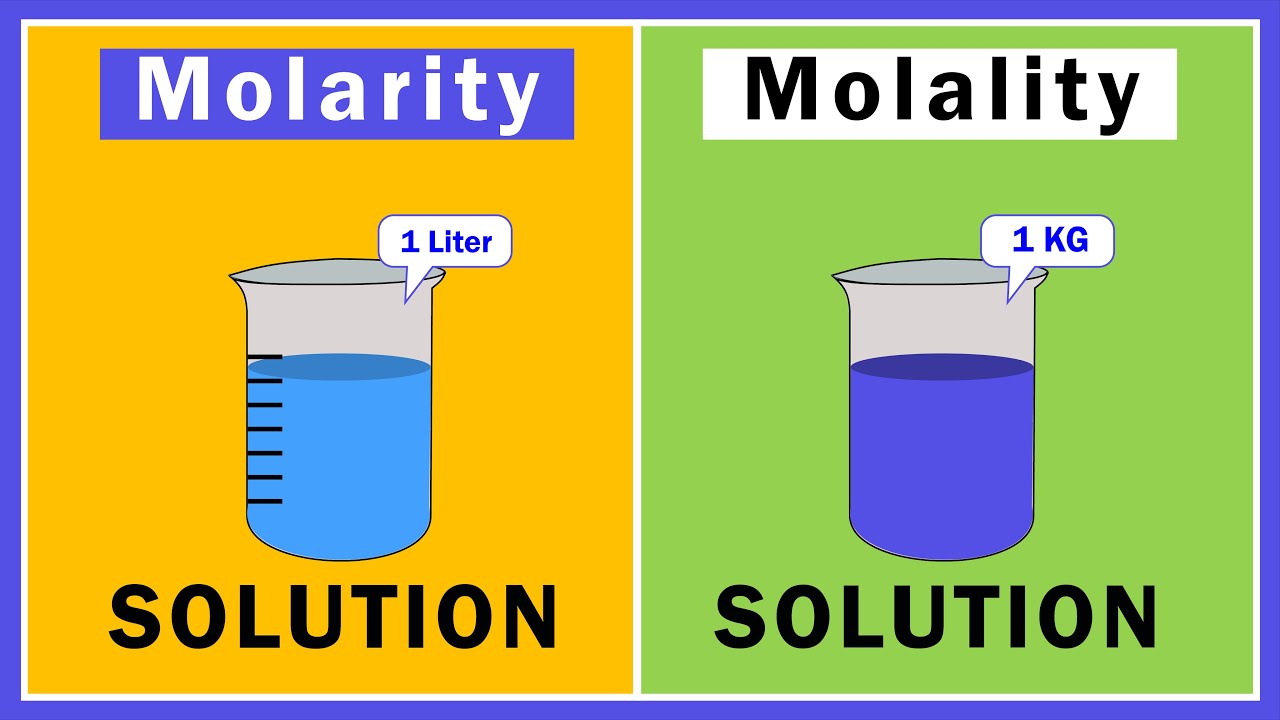
- 🔍 Marity is defined as the number of moles of solute divided by the liters of solution, which includes both solute and solvent.
- 📚 Molality is the number of moles of solute divided by the kilograms of solvent, focusing on the mass of the solvent alone.
- 🌡 One subtle but important difference is that marity considers the combined volume of solute and solvent, while molality considers only the mass of the solvent.
- 🧪 Making a molar solution involves dissolving a certain mass of solute in 1 liter of solution, whereas a molal solution involves dissolving the same mass in 1 kilogram of solvent.
- 💡 The process of making a molar solution requires measuring the volume to ensure it reaches 1 liter, while molality only requires weighing the solute and solvent.
- 🤔 It's a common misconception that molar and molal concentrations will be similar because 1 liter of water weighs 1 kilogram, but this is not true for all solvents.
- 🌟 Molality has an advantage over molarity in that it does not require precise volume measurements, making it simpler to prepare solutions.
- 🧐 There are technical differences between molar and molal solutions that can be explored further in additional educational content.
- 📈 Understanding the distinction between molarity and molality is crucial for accurately preparing and analyzing solutions in chemistry.
Q & A
What are marity and molality?
-Marity and molality are both measures of concentration that describe the amount of solute dissolved in a certain amount of solution.
How is marity defined?
-Marity is defined as the number of moles of solute divided by the liters of solution, which includes both the solute and solvent combined.
What is the definition of molality?
-Molality is defined as the number of moles of solute divided by the kilograms of solvent, focusing on the mass of the solvent alone.
What is the key difference between marity and molality?
-The key difference is that marity is concerned with the volume of the solution (liters), while molality is concerned with the mass of the solvent (kilograms).
Why might marity and molality seem similar when dealing with water?
-They might seem similar because one liter of water weighs approximately 1 kilogram, making the numerical values of molarity and molality close when dealing with water as a solvent.
What is the practical difference when making a molar solution compared to a molal solution?
-When making a molar solution, you dissolve the solute in a specific volume of solution until it reaches one liter. For a molal solution, you dissolve the solute in a specific mass of solvent (1 kilogram), and the final volume is not as critical.
What is an advantage of molality over molarity when preparing a solution?
-An advantage of molality is that you only need a scale to weigh out the solute and solvent, making it simpler than molarity, which requires measuring the volume precisely in a flask.
Why might textbooks and teachers emphasize the difference between marity and molality?
-They emphasize the difference because it is a common point of confusion, and understanding the distinction is crucial for accurately preparing and understanding solutions.
What is the molar mass of calcium chloride mentioned in the script?
-The molar mass of calcium chloride mentioned in the script is 111.0 grams.
How does the preparation of a one molar solution of calcium chloride differ from a one molal solution?
-For a one molar solution, you dissolve 111 grams of calcium chloride in 1 liter of solution. For a one molal solution, you dissolve 111 grams of calcium chloride in 1 kilogram of water.
What is the importance of understanding the difference between marity and molality in chemistry?
-Understanding the difference is important because it affects how solutions are prepared, how their concentrations are measured, and their behavior in various chemical reactions and processes.
Outlines
🧪 Understanding Molarity and Molality
This paragraph delves into the concepts of molarity and molality, two measures of concentration in chemistry. Molarity is defined as the moles of solute per liter of solution, which includes both the solute and solvent. Molality, on the other hand, is the moles of solute per kilogram of solvent. The distinction between them is crucial as it affects how solutions are prepared and understood. The speaker illustrates the difference by explaining how to prepare a molar and a molal solution of calcium chloride, emphasizing the procedural differences and the importance of understanding the composition of the solution in terms of volume versus mass.
🔍 Further Exploration of Molarity and Molality
The second paragraph serves as a teaser for a follow-up video that will explore solutions and the specific points of interest regarding molality. It suggests that there are additional technical differences between molarity and molality that will be discussed in more detail, inviting viewers to stay tuned for further insights into these chemical concepts.
Mindmap
Keywords
💡Marity
💡Molality
💡Solute
💡Solvent
💡Concentration
💡Molar Mass
💡Volumetric Flask
💡Scale
💡Molar Solution
💡Molal Solution
💡Technical Differences
Highlights
Marinity and molality are both measures of concentration, but they have important differences.
Marinity is defined as the number of moles of solute divided by the liters of solution.
Molality is the moles of solute divided by the kilograms of solvent.
Marinity is about liters of solution, which includes both solute and solvent.
Molality is about the mass of the solvent alone.
Making a molar solution involves dissolving a specific amount of solute in 1 liter of solution.
Creating a molal solution requires dissolving a specific amount of solute in 1 kilogram of water.
The process of making a molar solution involves using a volumetric flask and marking the one-liter line.
For a molal solution, you weigh out the solute and solvent separately and then mix them.
One liter of water weighs 1 kilogram, which can cause confusion between molarity and molality.
Molality is advantageous because it only requires a scale to measure solute and solvent, not a volumetric flask.
Molarity and molality differ significantly when using solvents other than water.
Molality is useful for solutions where the volume can fluctuate with temperature.
Technical differences between molarity and molality solutions will be discussed in a follow-up video.
Molarity is commonly used to measure concentration, but molality offers practical benefits.
Understanding the differences between molarity and molality is crucial for accurate chemical calculations.
Transcripts
Browse More Related Video
What is molarity and molality Class 11? | What is molality and example? | calculate molality

Molarity vs. molality | Lab values and concentrations | Health & Medicine | Khan Academy

Molarity versus Molality

Difference between Molarity and Molality

How to Calculate Molality

What's the Point of Molality?!?
5.0 / 5 (0 votes)
Thanks for rating: