8.6 Halogenation of Alkenes and Halohydrin Formation | Organic Chemistry
TLDRThis lesson delves into the intricacies of halogenation and halohydrin formation, two critical reactions in organic chemistry. Halogenation involves the addition of halogens like Cl2, Br2, or I2 to an alkene in an inert solvent, resulting in a reaction with no regioselectivity due to the symmetric addition. The process is stereoselective, favoring an anti-addition mechanism and avoiding carbocation intermediates, thus preventing rearrangements. Halohydrin formation, contrastingly, occurs in a reactive solvent like water or an alcohol, leading to Markovnikov's rule adherence and the creation of a halogen-oxygen or halogen-carbon bond on the alkene. The lesson emphasizes the importance of stereochemistry, particularly when two chiral centers are formed, and illustrates the mechanisms behind these reactions, including the formation of three-membered rings with halogens, known as bromonium, chloronium, or halonium ions. The instructor also discusses the role of solvents in influencing the reaction's outcome, highlighting how water or alcohol can participate in the reaction, leading to different products. The summary encourages engagement with the content and the channel, inviting viewers to subscribe for weekly lessons throughout the 2020-21 school year and interact through likes, shares, and comments.
Takeaways
- 🌟 Halogenation involves adding a halogen like Cl2, Br2, or I2 across an alkene in an inert solvent, resulting in no regioselectivity due to the addition of two identical halogens.
- 🔍 The stereoselectivity of halogenation is anti-addition, which means the addition occurs from the opposite side of the nucleophile, and it does not proceed through a carbocation intermediate, thus avoiding rearrangements.
- ⚙️ Halohydrin formation is similar to halogenation but occurs in a reactive solvent like water or alcohol, leading to the addition of a halogen and an OR or OH group across the alkene.
- 📍 In halohydrin formation, Markovnikov's rule applies, where the halogen adds to the less substituted carbon, and the OH or OR group adds to the more substituted carbon, resulting in anti-addition.
- 💡 The mechanism of halohydrin formation involves the formation of a three-membered ring with the halogen (e.g., bromonium ion), followed by backside attack by the solvent molecule.
- ⚖️ When two chiral centers are formed in a reaction, the stereochemistry becomes significant, and the product can be a pair of enantiomers, depending on the orientation of the groups added.
- 🔬 The solvent used in the reaction can influence the outcome; in the case of halohydrin formation, the solvent (water or alcohol) acts as a nucleophile and participates in the reaction.
- 🧬 The presence of a chiral center in a molecule is crucial for determining stereoisomers; different representations of the chiral center result in different stereoisomers.
- 🛑 The absence of a carbocation intermediate in both halogenation and halohydrin formation reactions means that no carbocation rearrangements occur, simplifying the product prediction.
- 📚 Students often confuse Markovnikov's rule; it's essential to understand that it refers to the regioselectivity of the addition, not the addition of hydrogen across the alkene.
- 🔬 The term 'anti-addition' refers to the addition of reagents from opposite faces of the molecule, which is a common theme in both halogenation and halohydrin formation reactions.
Q & A
What is the key difference between halogenation and halohydrin formation in terms of the solvent used?
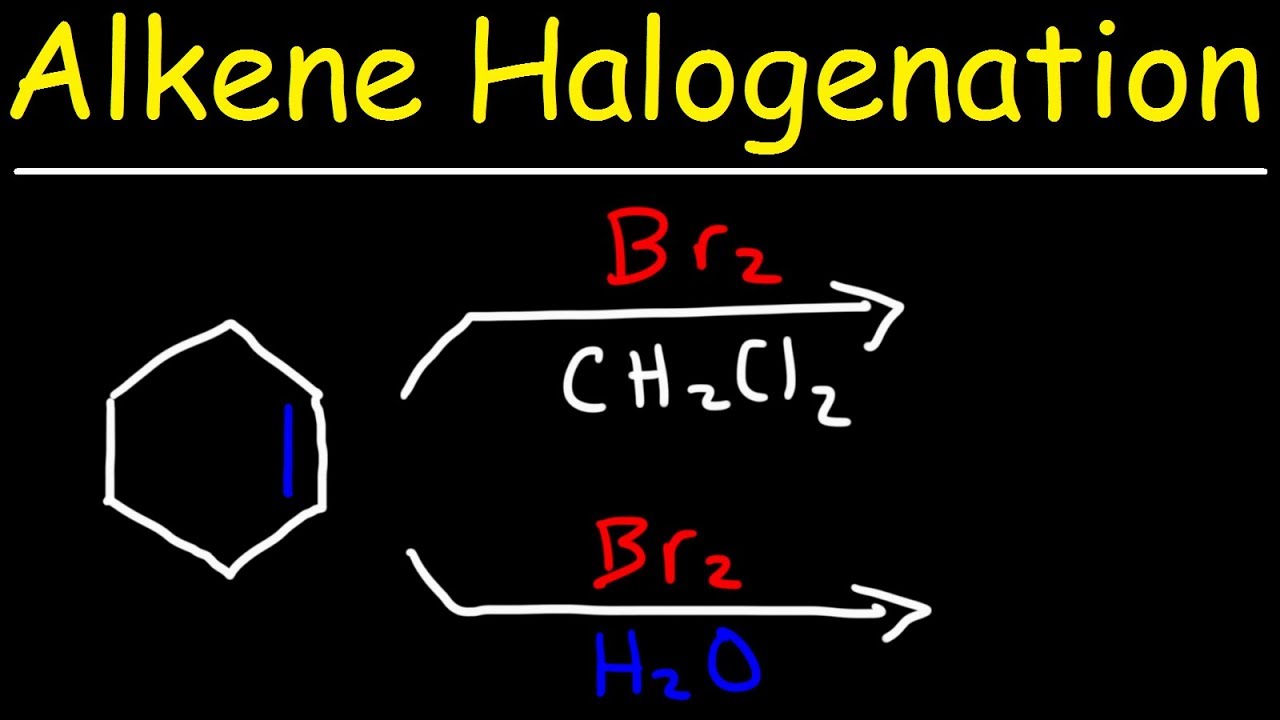
-Halogenation is carried out in an inert solvent like carbon tetrachloride (CCl4) or dichloromethane (CH2Cl2), while halohydrin formation is performed in a reactive solvent such as water or an alcohol.
Why does the stereoselectivity matter when forming two chiral centers in a reaction?
-Stereoselectivity matters because it determines the spatial arrangement of the atoms in the molecule. When two chiral centers are formed, there are four possible stereoisomers (two diastereomers and two enantiomers), and the reaction will typically form only two of these (the anti-addition products).
What is the term used to describe the intermediate formed when a halogen reacts with an alkene without forming a carbocation?
-The intermediate is called a halonium ion, which can be specifically a bromonium ion, chloronium ion, or a more generic term, a halonium ion, depending on the halogen involved.
Why does the Markovnikov rule apply in halohydrin formation but not in halogenation?
-In halohydrin formation, the reaction involves the addition of a halogen and a hydroxyl or alkoxy group from water or an alcohol, respectively. The Markovnikov rule applies because the electronegative halogen gets added to the less substituted carbon (electron-deficient), and the hydroxyl or alkoxy group gets added to the more substituted carbon.
What is the reason for the anti-addition observed in both halogenation and halohydrin formation reactions?
-The anti-addition is due to the backside attack of the nucleophile on the sp3 hybridized carbon atom in the intermediate. This backside attack is facilitated by the three-membered ring intermediate (halonium ion) and results in the addition of the nucleophile and the leaving group on opposite faces of the molecule.
How does the presence of a reactive solvent in halohydrin formation affect the reaction mechanism?
-In halohydrin formation, the reactive solvent (water or alcohol) acts as a weak nucleophile and preferentially performs the backside attack on the halonium ion, leading to the opening of the three-membered ring and the formation of the halohydrin product.
What is the role of the bromonium ion in the halogenation reaction?
-The bromonium ion is a three-membered ring intermediate formed when a bromine molecule reacts with an alkene. It is a key intermediate that facilitates the backside attack by a bromide ion, leading to the anti-addition of two bromine atoms across the alkene.
Why are wedges and dashes important when representing chiral centers in a molecule?
-Wedges and dashes are used to represent the three-dimensional configuration of chiral centers. They indicate the stereochemistry, showing whether the groups are in the front (wedge) or back (dash) of the plane of the paper, which is crucial for distinguishing between different stereoisomers.
What is the significance of the term 'enantiomer' in the context of stereochemistry?
-Enantiomers are stereoisomers that are mirror images of each other but are not identical, much like left and right hands. They have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional arrangement of those atoms in space.
How does the mechanism of halohydrin formation differ when an alcohol is used instead of water?
-When an alcohol is used instead of water, the hydroxyl group (OH) in the reaction products is replaced by an alkoxy group (OR), where R represents the alkyl group of the alcohol. This substitution does not change the overall mechanism but alters the specific groups attached to the final product.
What is the role of the solvent in determining the product distribution in halohydrin formation?
-The solvent, whether water or an alcohol, participates in the reaction and influences the product distribution. Due to the high concentration of solvent molecules, they preferentially perform the backside attack on the halonium ion, leading to the formation of halohydrin products with the halogen on the less substituted carbon and the solvent-derived group on the more substituted carbon.
Outlines
🌟 Halogenation and Halohydrin Formation Overview
This paragraph introduces the topic of halogenation and halohydrin formation. Halogenation involves the addition of halogens like Cl2, Br2, or I2 across an alkene in an inert solvent, leading to no regioselectivity due to the anti-addition mechanism and the absence of carbocation intermediates, thus preventing rearrangements. Halohydrin formation differs by taking place in water or alcohol, resulting in Markovnikov's rule application and also proceeding via an anti-addition without carbocation intermediates. The paragraph also mentions the creation of a new organic chemistry playlist with weekly lessons throughout the 2020-21 school year and encourages subscription for updates.
🔍 Mechanism of Halogenation and Stereoselectivity
The second paragraph delves into the mechanism of halogenation, emphasizing the nucleophilic attack of the alkene on the halogen to form a three-membered ring, such as a bromonium ion. It discusses the lack of carbocation formation and the resulting anti-addition, leading to specific stereochemical outcomes. The paragraph also explores the formation of chiral centers and the resulting stereoisomers, highlighting the importance of drawing both R and S enantiomers when one chiral center is formed and the generation of enantiomers when two chiral centers are created. The summary includes a brief explanation of the solvents typically used in these reactions, such as CCl4 and CH2Cl2.
🧪 Halohydrin Formation Mechanism and Markovnikov's Rule
The third paragraph focuses on the mechanism of halohydrin formation, contrasting it with halogenation by highlighting the participation of water or alcohol as reactive solvents. It explains how the reaction results in the addition of a halogen and either an OH or OR group across the alkene, following Markovnikov's rule. The paragraph clarifies misconceptions about Markovnikov's rule when no hydrogen is added across the alkene and illustrates how the solvent, due to its abundance, preferentially performs the backside attack, leading to the formation of a product with the halogen on the less substituted side and the solvent-derived group on the more substituted side. The summary also touches on the formation of enantiomers and the role of the solvent in determining the final product's stereochemistry.
Mindmap
Keywords
💡Halogenation
💡Halohydrin formation
💡Regioselectivity
💡Stereoselectivity
💡Markovnikov's rule
💡Anti-addition
💡Carbocation rearrangements
💡Chiral center
💡Enantiomers
💡Inert solvent
💡Bromonium ion
Highlights
Halogenation reaction involves adding a halogen such as Cl2, Br2, or I2 across an alkene without regioselectivity due to the addition of two identical halogens.
Stereoselectivity in halogenation is anti-addition, which does not lead to carbocation intermediates and thus no rearrangements are possible.
Halohydrin formation occurs when the reaction is carried out in water or an alcohol, leading to Markovnikov addition.
In halohydrin formation, a halogen and either an OH or OR group are added across the alkene, resulting in a different product from halogenation.
The mechanism of halohydrin formation involves a three-membered ring with a halogen, known as a bromonium, chloronium, or halonium ion.
Chiral centers formed in alkene reactions result in the formation of both R and S enantiomers.
When two chiral centers are formed, stereoselectivity is crucial, and the addition is anti, leading to two possible enantiomers.
The solvent used in halogenation can be inert like CCl4 or CH2Cl2, or reactive like water or alcohol, influencing the reaction outcome.
In the presence of a reactive solvent, the solvent molecules preferentially perform the backside attack in the mechanism of halohydrin formation.
Markovnikov's rule in the context of halohydrin formation implies that the halogen becomes attached to the less substituted carbon.
Students often misunderstand Markovnikov's rule due to its oversimplified 'rich get richer' mnemonic, which does not apply when no hydrogen is added across the alkene.
The final products of halohydrin formation are chiral centers that can exist as a pair of enantiomers, depending on the stereochemistry of the addition.
The mechanism of halohydrin formation involves the formation of a chloride ion and the backside attack by the solvent, which is a weak nucleophile compared to chloride.
In the case of using an alcohol instead of water, the mechanism would result in an OR group attaching where the OH group would have in the presence of water.
The channel provides weekly organic chemistry lessons throughout the 2020-21 school year, with the next lesson focusing on alkene addition reactions.
Subscribers to the channel are notified every time a new lesson is posted, ensuring they do not miss any updates.
The instructor, Chad, encourages viewers to subscribe, click the bell notification, and engage with the content through likes and shares.
For further study materials, including quizzes, chapter tests, and practice final exams, Chad recommends visiting his website, chadsprep.com.
Transcripts
Browse More Related Video

8.2 Hydrohalogenation of Alkenes | Organic Chemistry

8.4 Addition of an Alcohol | Acid-Catalyzed Addition and Alkoxymercuration-Demercuration | OChem
Halogenation of Alkenes & Halohydrin Formation Reaction Mechanism

8.1 Introduction to Alkene Addition Reactions; Markovnikov's Rule and Syn vs Anti | OChemistry

8.8 How to Predict the Products of Alkene Addition Reactions | Organic Chemistry

Hydroboration Oxidation of Alkenes Reaction and Mechanism: Alkene Vid 10
5.0 / 5 (0 votes)
Thanks for rating: