Stereochemistry: Crash Course Organic Chemistry #8
TLDRThis Crash Course Organic Chemistry episode dives into the fascinating world of stereochemistry, focusing on the importance of molecular shape in biological and chemical processes. Host Deboki Chakravarti explains how slight geometric differences can significantly impact a molecule's function, using the example of D-glucose and L-glucose to illustrate the concept. The video explores various types of isomers, including constitutional and geometric isomers, and introduces the concept of chirality and enantiomers. It also covers the Cahn-Ingold-Prelog convention for naming enantiomers and provides a step-by-step guide on how to assign R and S labels to chiral centers. The episode concludes with a discussion on cyclic compounds and a flowchart to help viewers determine whether a molecule is chiral or achiral, setting the stage for future lessons on the properties of enantiomers and their interaction with light.
Takeaways
- 🚀 Dr. Gilbert Levin considered the possibility of Martian life preferring a different form of glucose (L-glucose) compared to Earth life (D-glucose), which led to the inclusion of both in the Viking lander samples sent to Mars.
- 🍬 L-glucose tastes the same as D-glucose but cannot be digested by humans, meaning it wouldn't convert into energy or be stored as fat, theoretically making it a perfect sugar substitute.
- 💸 The production of L-glucose is too expensive, which is why it never reached the market as a sugar substitute.
- 🔍 The concept of stereochemistry is crucial as it explains how the slight differences in the spatial arrangement of atoms in molecules can lead to different biological activities.
- 🤚 Isomers are molecules with the same molecular formula but different arrangements of atoms, which can be constitutional or geometric, affecting their properties and reactivity.
- 🕶️ Stereoisomers differ in the spatial relationships between atoms, even though they have the same atoms connected in the same sequence.
- 🖐 The concept of chirality arises when a molecule has a non-superimposable mirror image, often associated with a carbon atom bonded to four different groups, known as a chiral center.
- 🔑 The Cahn-Ingold-Prelog convention is used to label chiral centers as R (rectus, right-handed) or S (sinister, left-handed), based on the priority of the groups attached to the chiral center.
- 📈 Assigning priorities to the groups around a chiral carbon involves considering the atomic number, with the lowest priority usually being a hydrogen atom.
- 🔬 Enantiomers, which are non-superimposable mirror images of each other, can have different effects in biological systems, which is significant in the pharmaceutical industry.
- ⚙️ Molecules with two chiral centers can be either chiral or achiral, depending on whether they have a superimposable mirror image or an internal plane of symmetry.
- 🌟 Stereochemistry plays a vital role in understanding molecular interactions, particularly important for the development of drugs with specific therapeutic effects.
Q & A
What was Dr. Gilbert Levin's initial motivation for including D-glucose and L-glucose in the samples sent to Mars on the Viking lander?
-Dr. Gilbert Levin included D-glucose and L-glucose in the samples to determine if Martian life would consume the same type of sugar as life on Earth, or if it preferred the 'left-handed' version, L-glucose.
Why can't humans digest L-glucose?
-Humans cannot digest L-glucose because our enzymes do not recognize it, which means it is not converted into energy or stored as fat.
Why did L-glucose not become a marketable sugar substitute despite its potential as a sweetener?
-L-glucose was too expensive to produce, which prevented it from becoming a viable sugar substitute on the market.
What is the significance of stereochemistry in understanding how the right-handed and left-handed versions of glucose affect biological processes?
-Stereochemistry is crucial because it explains how small geometric differences in molecules, such as the right-handed D-glucose and left-handed L-glucose, can lead to different interactions with biological systems.
What is a chiral center in chemistry?
-A chiral center is a carbon atom with four different groups attached to it, which gives rise to non-superimposable mirror images known as enantiomers.
How do you determine if a molecule is chiral or achiral?
-A molecule is chiral if it has a non-superimposable mirror image and does not have an internal plane of symmetry. If it has a superimposable mirror image or an internal plane of symmetry, it is achiral.
What is the Cahn-Ingold-Prelog convention used for in organic chemistry?
-The Cahn-Ingold-Prelog convention is used to label the chiral centers of a molecule as R (for right-handed) or S (for left-handed) based on the priority of the groups attached to the chiral center.
How do you assign priority to the groups around a chiral carbon for naming enantiomers?
-Priority is assigned based on the atomic number of the atoms in the groups attached to the chiral carbon, with the highest atomic number getting the highest priority.
What is the difference between a cis isomer and a trans isomer in molecules with double bonds?
-In a cis isomer, the attached groups are on the same side of the double bond, while in a trans isomer, the groups are on opposite sides of the double bond.
Why is it important to practice drawing enantiomers in organic chemistry?
-Practicing drawing enantiomers helps in understanding the spatial arrangement of atoms in molecules, which is essential for predicting their interactions with biological systems and their potential effects.
How do the properties of enantiomers differ, and why is this significant in the context of pharmaceuticals?
-Enantiomers can have different biological activities; one enantiomer may be an effective medication, while the other might have a different effect or be inactive. This is significant in pharmaceuticals because it can affect the safety and efficacy of drugs.
What is the role of the Crash Course Organic Chemistry app in enhancing the learning experience for students?
-The Crash Course Organic Chemistry app allows students to review content from the series, providing a platform for further study and enhancing their understanding of organic chemistry concepts.
Outlines
🔍 Introduction to Stereochemistry and Chirality
The video begins with a brief introduction to the Crash Course Organic Chemistry app and the host, Deboki Chakravarti. It delves into the story of Dr. Gilbert Levin's experiment to detect life on Mars by sending D-glucose and L-glucose to see if Martian life would consume either. The video explains that while D-glucose is used by most life on Earth for energy, L-glucose, despite tasting the same, is not digestible by humans. This leads to a discussion about the importance of molecular shape, specifically stereochemistry, in determining how molecules interact with life. The video covers constitutional and geometric isomers, the concept of chirality, and the identification of chiral centers in molecules. It also introduces the concept of enantiomers and how to draw them, using butan-2-ol and albuterol as examples.
📜 Cahn-Ingold-Prelog Convention and Enantiomer Nomenclature
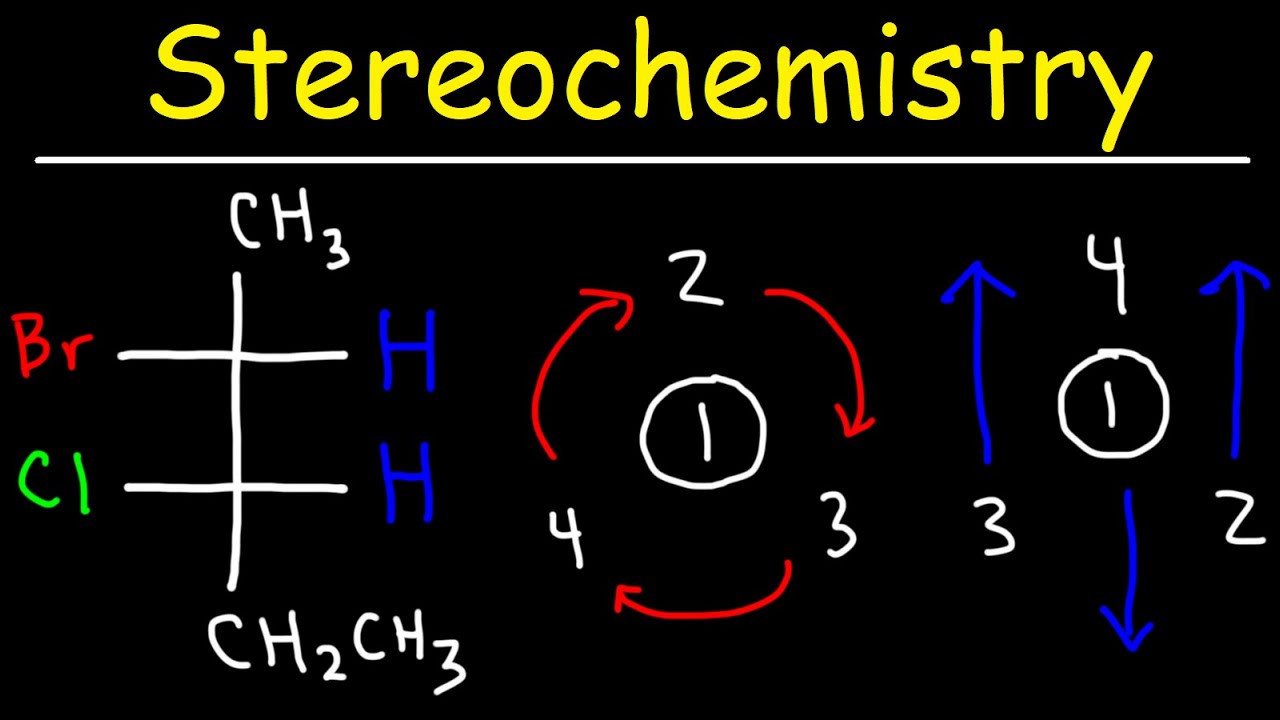
This paragraph focuses on the Cahn-Ingold-Prelog (CIP) convention used to name enantiomers. It explains the process of assigning priorities to the groups around a chiral carbon based on atomic numbers, with hydrogen typically having the lowest priority. The video demonstrates how to assign R or S configurations to chiral centers using the CIP rules, taking into account the spatial arrangement of the highest to lowest priority groups. It uses butan-2-ol and albuterol to illustrate the process of naming enantiomers, highlighting the importance of considering the lowest priority group's orientation for consistency in naming.
🧬 Chirality in Cyclic Compounds and Molecules with Multiple Chiral Centers
The final paragraph explores chirality in cyclic compounds, such as methylcyclopentane, which lacks an internal plane of symmetry and is therefore achiral. It contrasts this with 2-methylcyclopent-1-ene, which is chiral due to the absence of an internal plane of symmetry. The video explains how to assign priorities and name enantiomers for molecules with a single chiral center and extends this to molecules with multiple chiral centers, like 4-bromohexan-3-ol. It also discusses how some molecules with multiple chiral centers can be achiral if they possess an internal plane of symmetry, using cis- and trans-1,2-dibromocyclohexane as examples. The video concludes with a flowchart to help viewers determine if a molecule is chiral or achiral and a teaser for the next episode, which will cover the properties of enantiomers, their interaction with light, and methods for their separation.
Mindmap
Keywords
💡Organic Chemistry
💡Stereochemistry
💡Isomers
💡D-glucose and L-glucose
💡Enantiomers
💡Chirality
💡Cahn-Ingold-Prelog (CIP) Convention
💡cis and trans Isomers
💡Achiral Molecules
💡Chiral Centers
💡Albuterol
Highlights
Dr. Gilbert Levin, an American engineer, considered the possibility of Martian life preferring a different type of sugar than life on Earth, leading to the inclusion of both D-glucose and L-glucose in the Viking lander's Mars samples.
L-glucose, the 'left-handed' sugar, was found to have the same taste as D-glucose but is indigestible to humans due to a lack of enzyme recognition.
Theoretically, L-glucose could be a sugar substitute without energy conversion or fat storage, but its production is too expensive for market use.
The difference in functionality between D-glucose and L-glucose is attributed to stereochemistry, which is the study of the spatial arrangement of atoms in molecules.
Isomers are molecules with the same molecular formula but different arrangements of atoms, with constitutional isomers having atoms connected in different ways.
Geometric isomers are a type of stereoisomer found in molecules with double bonds, where the attached groups can be arranged differently, resulting in cis or trans isomers.
Stereoisomers differ in the spatial relationships between atoms, even though they have the same atoms connected in the same sequence.
Chirality in chemistry refers to a molecule having a non-superimposable mirror image, such as a carbon atom with four different groups attached.
Enantiomers are non-superimposable mirror images of a chiral molecule, derived from a chiral center or a carbon with four different groups attached.
The Cahn-Ingold-Prelog convention is used to label chiral centers as R (right-handed) or S (left-handed) based on the priority of the groups attached to the chiral carbon.
Assigning priorities to groups around a chiral carbon involves considering the atomic number of the atoms, with the lowest priority usually being a hydrogen atom.
The priority of groups is determined by the atomic number, and the spatial arrangement of these groups helps in naming the enantiomers as R or S.
Cyclic compounds like methylcyclopentane can be achiral if they possess an internal plane of symmetry, meaning their mirror images can be superimposed.
Molecules with two chiral centers can be achiral if they have a superimposable mirror image or an internal plane of symmetry, as seen in cis-1,2-dibromocyclohexane.
A flowchart is provided to help determine if a molecule is chiral or achiral by assessing the presence of chiral centers and internal planes of symmetry.
Stereochemistry plays a critical role in understanding molecular interactions, particularly in the context of drug development and their effects.
The episode concludes with a teaser for the next installment, which will explore the properties of enantiomers, their interaction with light, and methods for their separation.
Transcripts
Browse More Related Video

More Stereochemical Relationships: Crash Course Organic Chemistry #9

5.2 How to Assign R and S | Absolute Configuration | Organic Chemistry

5.1 Overview of Isomers | Constitutional Isomers and Stereoisomers | Organic Chemistry

Stereochemistry: Meso Compounds, Diastereomers

5.5 How to Identify Type of Isomerism | Organic Chemistry
Stereochemistry - R S Configuration & Fischer Projections
5.0 / 5 (0 votes)
Thanks for rating: