Micro vs Macro States
TLDRThe video script delves into the fundamental difference between microstates and macrostates, a crucial distinction for understanding the transition from statistical mechanics to thermodynamics. Microstates, which encompass all detailed information about a system's particles, such as their positions, velocities, and energies, are contrasted with macrostates, which provide a more general description focusing on large-scale properties like pressure, volume, and temperature. The script uses the analogy of coin flips to illustrate the concept, showing how multiple microstates can correspond to a single macrostate. It emphasizes that while microstates offer a complete picture, macrostates are often more practical, especially for systems with numerous particles where tracking individual details is not feasible. This understanding is vital for grasping complex concepts like entropy and the behavior of thermodynamic systems.
Takeaways
- 🔍 **Microstates vs. Macrostates**: The distinction between microstates and macrostates is crucial for understanding the transition from statistical mechanics to thermodynamics.
- 📏 **Microstate Description**: A microstate is a detailed description of a system's state, including the positions, velocities, and individual energies of all particles.
- 🌐 **Macrostate Description**: A macrostate provides a more coarse-grained view, focusing on large-scale properties like pressure, temperature, and volume, rather than individual particle details.
- 🪜 **From Micro to Macro**: Knowing the microstate of a system allows you to deduce its macrostate, but not vice versa, as the macrostate lacks the detailed information of the microstate.
- 🤝 **Multiple Microstates per Macrostate**: There are usually many microstates associated with a single macrostate, indicating a loss of detailed information when only the macrostate is known.
- 🔢 **Example of Coins**: Flipping coins illustrates the concept of microstates (each coin's head or tail) and macrostates (the total number of heads or tails).
- ⚖️ **Statistical Relevance**: In systems with many particles, like an ideal gas, tracking every microstate is impractical, making macrostates more useful for statistical analysis.
- 🔄 **Dynamic Microstates**: Microstates can change rapidly from moment to moment, while macrostates, especially in equilibrium, remain constant over time.
- 🎢 **Importance of Macrostates**: Macrostates are often more useful for describing systems with many degrees of freedom, as they provide an overview that is less sensitive to the system's rapid microscopic changes.
- 🔑 **Key to Thermodynamics**: Understanding microstates and macrostates is essential for grasping concepts like entropy and other thermodynamic principles that emerge from microscopic interactions.
- ⚙️ **Practicality in Large Systems**: For systems with a vast number of particles, such as Avogadro's number, macrostate descriptions are not only sufficient but more practical due to the complexity of microstate tracking.
Q & A
What is the primary difference between microstates and macrostates?
-Microstates contain all the information about a system's state, including the positions, velocities, and energies of individual particles. Macrostates, on the other hand, provide a more coarse-grained description focusing on large-scale properties like pressure, temperature, and volume.
Why is the concept of microstates and macrostates important in statistical mechanics?
-The concept is crucial for understanding the transition from statistical mechanics to thermodynamics, as it helps to comprehend how macroscopic properties of a system emerge from the behavior of its microscopic components.
How does the description of an ideal gas using microstates differ from using macrostates?
-A microstate description of an ideal gas would involve tracking every particle's velocity, position, and energy. In contrast, a macrostate description would focus on overall properties such as volume, pressure, and temperature, which are more stable and less detailed.
What is an example of a microstate in the context of the coin flip scenario?
-A microstate in the coin flip scenario is a specific outcome of the coin tosses, such as 'Heads-Heads-Tails' (HHT), where the exact position of each coin's result is known.
What is an example of a macrostate in the context of the coin flip scenario?
-A macrostate in the coin flip scenario would be a summary that counts the number of heads and tails without specifying their order, such as '2 heads and 1 tail'.
How does the number of microstates relate to the number of macrostates for a given system?
-There are generally multiple microstates associated with each macrostate. This is because a macrostate is a summary that doesn't include the specifics of each microstate, thus grouping many possible microstates under a single macrostate.
Why might one prefer to use macrostate descriptions over microstate descriptions in certain situations?
-Macrostate descriptions are often more useful when dealing with systems with many degrees of freedom, such as an ideal gas with Avogadro's number of particles, because tracking every single particle's state is impractical. Macrostates provide a more manageable level of detail that is often sufficient for understanding large-scale behavior.
What are some examples of macrostate properties for an ideal gas?
-Examples of macrostate properties for an ideal gas include pressure, temperature, volume, and total internal energy. These properties describe the system on a large scale and are typically constant in an equilibrium state.
What is the significance of the microstate description changing from second to second in an isolated system?
-The constant change in the microstate description, while the macrostate remains constant, illustrates the distinction between the microscopic dynamics and the macroscopic stability in an isolated system. It shows that individual particle interactions and movements lead to no change in the observable, large-scale properties over time.
How does the concept of microstates and macrostates help in understanding entropy?
-The concept helps in understanding entropy by showing how the disorder or randomness at the microscopic level (many microstates) corresponds to macroscopic measures of disorder, which is entropy. The higher the number of microstates associated with a macrostate, the greater the entropy.
Why is the microstate description considered to contain 'all of the information' about a system's state?
-The microstate description is considered to contain all the information because it specifies the exact state of each individual component in the system, such as the position and velocity of every particle. This level of detail provides a complete picture of the system at a given moment.
What is the role of Avogadro's number in the context of discussing microstates and macrostates?
-Avogadro's number, which represents the number of particles in one mole of a substance, is used to illustrate the impracticality of tracking individual microstates in large systems like an ideal gas. It emphasizes why macrostate descriptions are often more useful for such systems due to the sheer number of particles involved.
Outlines
📚 Introduction to Micro and Macro States
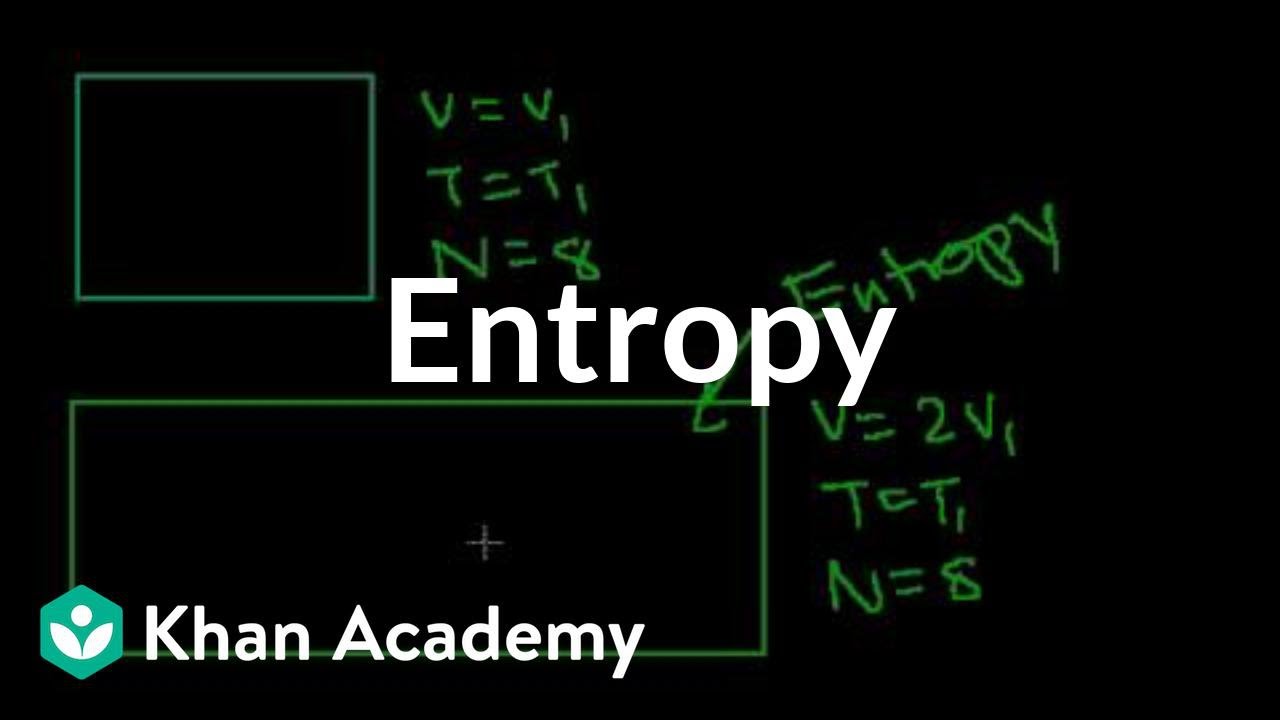
The first paragraph introduces the concepts of micro and macro states, which are crucial for understanding the transition from statistical mechanics to thermodynamics. A microstate describes a system at the microscopic level, detailing the positions, velocities, and energies of all individual particles. In contrast, a macrostate provides a more coarse-grained description, focusing on large-scale properties like the system's overall structure. The paragraph uses the example of flipping three coins to illustrate microstates, where each coin's outcome (head or tail) represents a different state, and macrostates, which only count the number of heads and tails without specifying their order.
🔍 Macro States and Their Coarse-Grained Nature
The second paragraph delves deeper into the concept of macro states, emphasizing their coarse-grained nature compared to microstates. It explains that macro states lose some information about the specific arrangement of a system's components, which is acceptable when dealing with a large number of particles, such as in an ideal gas. The paragraph also discusses how macro states like pressure, temperature, and volume can describe an ideal gas without needing to track every particle's velocity or position. It highlights that multiple microstates can correspond to a single macrostate, making macro descriptions more manageable and often sufficient for understanding the system's behavior.
🌟 Microstates vs. Macrostates: Key Distinctions
The third paragraph summarizes the key differences between microstates and macrostates. It reiterates that microstates contain all the information about a system's state, while macrostates offer a less detailed, large-scale view. The paragraph also points out that there are usually multiple microstates associated with a single macrostate, especially in systems with many degrees of freedom, such as an ideal gas. It concludes by stressing the practicality of macrostates in describing systems where the microstate is constantly changing due to particle interactions, while the macrostate remains relatively constant, particularly in equilibrium states.
Mindmap
Keywords
💡Microstates
💡Macrostates
💡Statistical Mechanics
💡Thermodynamics
💡Ideal Gas
💡Avogadro's Number
💡Entropy
💡Particle Spins
💡Coarse-grained Description
💡Degrees of Freedom
💡Equilibrium State
Highlights
The distinction between microstates and macrostates is crucial for understanding the transition from statistical mechanics to thermodynamics.
Microstates provide a detailed description containing all information about a system's state, including particle positions, velocities, and individual energies.
Macrostates offer a coarse-grained description focusing on large-scale structures or properties such as pressure, temperature, and volume.
An example used to illustrate the concepts is the flipping of three coins, where microstates account for each coin's outcome, and macrostates summarize the total number of heads or tails.
Microstates and macrostates can also be applied to particle spins, with heads and tails analogous to spin up and spin down states.
The macrostate description is less detailed but often sufficient when dealing with a large number of particles, such as in an ideal gas.
For an ideal gas, microstate properties include individual particle velocities and positions, while macrostate properties include pressure, temperature, and volume.
Knowing the microstate of a system allows one to deduce the macrostate properties, but not vice versa.
Multiple microstates can correspond to a single macrostate, indicating a loss of specific information when only the macrostate is known.
In systems with many degrees of freedom, such as an ideal gas with numerous particles, the macrostate description is often more useful and practical.
The macrostate can remain constant over time, even when the microstate changes continuously, making it a more stable descriptor for large systems.
Understanding microstates and macrostates is fundamental for grasping concepts like entropy and other thermodynamic principles.
The microstate description changes from moment to moment in an isolated system, unlike the macrostate which may remain constant in equilibrium.
Macrostates are particularly useful for describing the state of a system when tracking individual particle details is not necessary or feasible.
The concept of macrostates allows for the simplification of complex systems into more manageable and understandable properties.
The lecture emphasizes the importance of this distinction for students aiming to understand statistical mechanics and its relation to thermodynamics.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: