More on entropy | Thermodynamics | Physics | Khan Academy
TLDRThis educational video script delves into the concept of entropy, emphasizing its role as a macrostate variable rather than a property of individual microstates. The instructor clarifies that entropy is not about specific arrangements of particles but the range of possible configurations a system can adopt. Using examples like a box with a divider and a deck of cards, the script illustrates how entropy increases with system size and temperature, highlighting the importance of understanding entropy as a measure of a system's disorder at the macro level.
Takeaways
- 📚 Entropy is a macrostate variable, meaning it describes the system's properties at a large scale rather than individual particle behavior.
- 🔍 The script emphasizes the importance of distinguishing between macrostates and microstates, with entropy being a property of the former.
- 💡 Entropy is not defined for individual microstates, such as a specific arrangement of molecules, but for the system as a whole in equilibrium.
- 🚫 Avoid the common misconception of assigning entropy to a particular state by looking at the arrangement of a few particles.
- 🔄 Entropy increases with the system's volume, assuming constant temperature and particle number, as it allows for more possible particle configurations.
- 📈 The script uses the example of a box with a divider to illustrate the concept of entropy and the transition from one macrostate to another.
- 🎯 Entropy is about the number of microstates a system can occupy, not the specific arrangement of objects at a macroscopic level.
- 🧩 The analogy of a clean versus a messy room is clarified to not be about the entropy of the room itself, but rather a misunderstanding of the concept.
- 🃏 The script points out that the entropy of a deck of cards is not about the order or disorder of the cards but the molecular vibrations within them.
- 🌡 Temperature is a macroscopic property that gives the average kinetic energy of particles, whereas entropy gives information about the system's possible states.
- 🔑 Entropy serves as a 'meta' state variable, summarizing the complexity of a system's microscopic states without needing to measure each one individually.
Q & A
What is the main focus of the video script?
-The main focus of the video script is to clarify the concept of entropy as a macrostate variable and to emphasize that it should not be confused with microstates.
Why does the script writer want to emphasize that entropy is a macrostate variable?
-The script writer wants to emphasize this because there is a common misunderstanding that entropy can be assigned to specific microstates, which is not accurate.
What is the difference between a macrostate and a microstate in the context of entropy?
-A macrostate refers to the overall properties of a system, such as volume, temperature, and pressure, which are only defined once the system is in equilibrium. A microstate, on the other hand, refers to a specific arrangement of particles within the system, which is not directly related to entropy.
Why is it incorrect to assign entropy to a particular microstate?
-It is incorrect because entropy is a measure of the number of possible microstates a system can be in, not a property of a single microstate. Entropy does not make sense for individual microstates.
What is the significance of the example with the box and divider in the script?
-The example with the box and divider illustrates the concept of entropy increase when a system goes from a confined state to a more open state, emphasizing that entropy is about the system's capacity to be in various states, not the specific arrangement of particles.
What does the script imply about the relationship between entropy and the cleanliness of a room?
-The script implies that the entropy of a room is not dependent on its cleanliness. It is an analogy to show that entropy is a macroscopic property, not something that can be visually assessed like dirtiness.
How does the script differentiate between the macrostates of a system of cards?
-The script differentiates macrostates by considering the system as a whole, not the specific arrangement of the cards. It emphasizes that both a neatly stacked and a messy pile of cards have the same entropy if they have the same mass and temperature.
What is the role of entropy in describing the states a system can take on?
-Entropy serves as a macrostate function that describes the number of possible microstates a system can be in, reflecting the system's disorder or randomness.
Why is it almost impossible to measure the microstates of a system?
-It is almost impossible to measure the microstates because particles at the atomic or molecular level are constantly vibrating and changing states due to thermal energy, making it impractical to track individual states.
What is the script's stance on using analogies like a clean or messy room to explain entropy?
-The script warns against using such analogies because they can lead to confusion and misunderstanding. Entropy is a macroscopic property and should not be associated with easily observable states like cleanliness.
How does the script describe the role of entropy in relation to other macrostate variables like temperature, pressure, and volume?
-The script describes entropy as a 'meta' macrostate variable that, like temperature, pressure, and volume, provides a shortcut for understanding the system's behavior without needing to measure the state of each individual molecule.
Outlines
🔍 Understanding Entropy as a Macrostate Variable
This paragraph emphasizes the concept of entropy as a macrostate variable, which is often misunderstood. The speaker clarifies that entropy is not about specific microstates but rather the macroscopic properties of a system. They use the example of a box with a divider to illustrate how entropy changes when the divider is removed, leading to a more even distribution of particles. The key point is that entropy is associated with the system as a whole, not individual states, and is defined once the system reaches equilibrium. The speaker also warns against the common mistake of assigning entropy to specific microstates, explaining that entropy is a measure of the number of possible states a system can be in, not the state itself.
📚 Entropy and Macrostates: Beyond Clean and Dirty Rooms
In this paragraph, the speaker further elaborates on the concept of entropy, dispelling the myth that it is related to the cleanliness or orderliness of a room. They argue that entropy is a macrostate variable that describes the number of possible states a system can take on, not the physical arrangement of objects. Using the analogy of a deck of cards, the speaker explains that whether the cards are neatly stacked or scattered does not affect their entropy. The true entropy is determined by the microscopic motion of the molecules, which is the same for both ordered and disordered systems. The speaker reiterates the importance of understanding entropy as a macroscopic property that simplifies the complexity of molecular motion, rather than focusing on the visible state of objects.
Mindmap
Keywords
💡Entropy
💡Macrostate
💡Microstate
💡Equilibrium
💡Volume
💡Temperature
💡Molecules
💡Macrostate Variable
💡Molecular Level
💡Kinetic Energy
💡States
Highlights
The video aims to clarify the concept of entropy as a macrostate variable, emphasizing its importance.
There is a common misconception about comparing entropy between microstates, which the video seeks to address.
Entropy is a property of the system as a whole, not individual microstates.
The video uses the classic example of a box with a divider to illustrate entropy concepts.
Macrostate variables like pressure, volume, temperature, and entropy are only defined at equilibrium.
The video demonstrates the concept of entropy increase when the divider is removed from the box.
Drawing molecules represents a microstate, not entropy, which is a property of the system.
The video explains that entropy does not make sense for individual microstates.
The entropy of a system is related to its volume, temperature, and number of particles.
The video emphasizes that entropy is a measure of the number of possible microstates a system can have.
An analogy of a clean versus dirty room is used to explain that entropy is not about the physical state but the system's macrostate.
The video clarifies that entropy is not about the physical arrangement of objects like cards, but the underlying molecular motion.
Entropy is a macroscopic property that describes the system's capacity to exist in various states.
The video explains that entropy is a shortcut to understand the complexity of molecular motion without measuring each molecule's state.
Entropy is presented as a 'meta' state variable, summarizing the system's potential microstates.
The video concludes by reiterating that entropy should not be confused with the physical appearance or arrangement of a system's components.
Transcripts
Browse More Related Video

Entropy of Free Expansion

Entropy intuition | Thermodynamics | Physics | Khan Academy
What is Entropy in Thermodynamics?
Reconciling thermodynamic and state definitions of entropy | Physics | Khan Academy

I don't believe the 2nd law of thermodynamics. (The most uplifting video I'll ever make.)
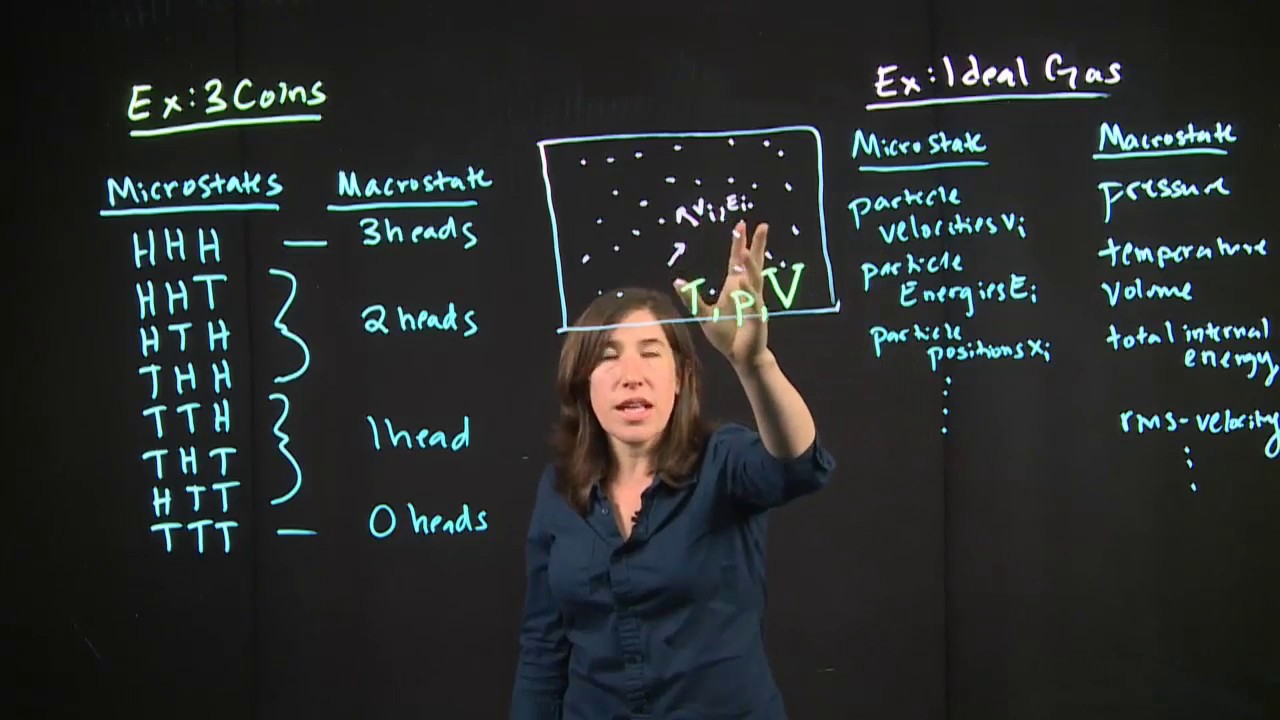
Micro vs Macro States
5.0 / 5 (0 votes)
Thanks for rating: