Modern Periodic Table
TLDRThis engaging video script takes viewers on a journey through the development of the modern periodic table, starting from its blank slate and progressively filling it with elements up to calcium, the 20th element. The video emphasizes the shift from Mendeleev's table, which was based on atomic mass, to the modern table that is organized by atomic number. It explains the significance of periods and groups, and how they relate to an element's electron configuration and chemical properties. The script also clarifies the electronic configurations of elements in groups 1 and 2, highlighting their valency and chemical similarities. The video further discusses the old and new group numbering schemes, introduces the color-coding system used in periodic tables to distinguish between different types of elements, and touches upon the special categories such as alkali metals, alkaline earth metals, halogens, and noble gases. It concludes with a nod to the modern periodic table's ability to correct anomalies of the past, including the placement of isotopes and elements like cobalt and nickel. The script encourages viewers to practice drawing the periodic table and to look forward to a future video on trends across periods and groups.
Takeaways
- 🔬 The modern periodic table is based on atomic number rather than atomic mass, which was the basis for Mendeleev's table.
- 📊 Atomic number is a more fundamental property for elements as it correlates more closely with their chemical properties than atomic mass.
- 🚀 The modern periodic table has seven periods (rows) and eighteen groups (columns), with the first four periods being the focus of the 'mini periodic table'.
- 🌟 The period number indicates the number of electron shells or orbits in an atom, which can be predicted without drawing the electronic structure.
- 💡 Elements in the same group have similar chemical properties and the same number of valence electrons, which is why they share the same valency.
- 📚 The electronic configuration of an element determines its chemical properties, with elements in the same group having similar configurations.
- 🔋 Alkali metals (Group 1) and alkaline earth metals (Group 2) are examples of groups with similar properties due to their electronic configurations.
- 🌈 The periodic table is often color-coded to represent different types of elements, such as metals, non-metals, metalloids, and noble gases.
- 📝 The old IUPAC group numbering scheme used Roman numerals and letters A and B, whereas the new scheme uses numbers 1 to 18.
- 🧲 Isotopes, which have the same atomic number but different mass numbers, fit nicely into the modern periodic table because they share the same chemical properties.
- 📉 The modern periodic table corrects anomalies in Mendeleev's table, such as the placement of elements like cobalt and nickel, which are now ordered by atomic number.
- ⚙️ The lanthanide and actinide series, which include rare earth and radioactive elements, are placed separately below the main body of the periodic table for compactness.
Q & A
What is the modern periodic table's primary basis for organizing elements?
-The modern periodic table is primarily organized based on atomic number, which is the number of protons present in the nucleus of an element.
Why is the atomic number more fundamental than atomic mass for classifying elements?
-The atomic number is more fundamental because it determines the chemical properties of elements more accurately than atomic mass, as demonstrated by the scientist Moseley.
What is the significance of the period number in the periodic table?
-The period number indicates the number of electron shells or electron orbits present in an atom, which allows us to predict the electronic structure without actually drawing it.
How does the number of valence electrons affect the chemical properties of elements?
-The number of valence electrons determines the chemical properties of elements because these electrons are involved in chemical bonding and reactions.
Why are elements in the same group of the periodic table said to have similar chemical properties?
-Elements in the same group have similar chemical properties because they have the same number of valence electrons, which are responsible for their chemical behavior.
What is the difference between the old and new group numbering schemes in the periodic table?
-The old scheme uses Roman numerals and the letters 'a' and 'b', while the new scheme uses numbers from 1 to 18. The new scheme is easier to remember, but it's important to know the old one as it is sometimes still used.
Why is hydrogen often given special treatment in the periodic table?
-Hydrogen is given special treatment because it exhibits properties similar to both alkali metals (Group 1) and halogens (Group 17), making its placement in the periodic table a subject of debate.
What are the lanthanide and actinide series, and why are they placed separately in the periodic table?
-The lanthanide series (elements 57-71) and actinide series (elements 89-103) are placed separately to compact the periodic table. These are series of elements known as rare earth elements and radioactive elements, respectively.
How does the modern periodic table correct the anomalies found in Mendeleev's periodic table?
-The modern periodic table corrects anomalies by being based on atomic number rather than atomic mass, allowing for a more accurate representation of elements, including the proper placement of isotopes and elements like cobalt and nickel.
What is the significance of the electronic configuration in determining the valency and chemical properties of elements?
-The electronic configuration, specifically the number of valence electrons, is crucial in determining the valency and chemical properties of elements, as it dictates how elements will interact in chemical reactions.
What is the importance of practicing drawing the periodic table and learning the first 20 elements?
-Practicing drawing the periodic table and learning the first 20 elements helps solidify understanding of the table's structure and the properties of elements, making it easier to predict their behavior in chemical reactions.
Outlines
🔍 Introduction to the Modern Periodic Table
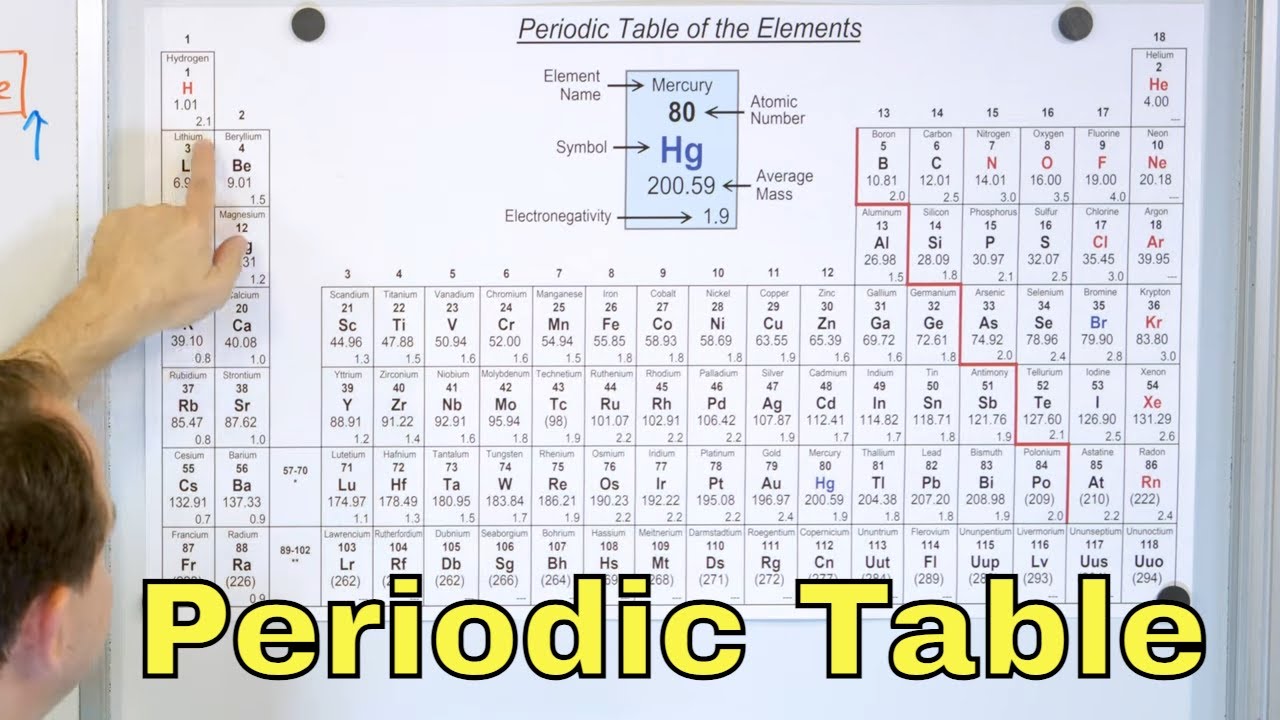
The video begins by setting the stage for the exploration of the modern periodic table, following previous discussions on earlier classification systems. The presenter proposes an interactive approach, starting with a blank table and progressively filling it with elements based on atomic numbers. The focus is on the first 20 elements, corresponding to the first four rows, creating a 'mini periodic table.' The audience is encouraged to draw this table to follow along. The video promises to cover the modern periodic table's structure, including rows (periods) and columns (groups), and the significance of atomic numbers, distinguishing it from Mendeleev's table which was based on atomic mass.
🌟 Understanding Periods and Groups
This paragraph delves into the concept of periods and groups in the periodic table. Periods, which are the rows, represent the number of electron shells an element has, allowing us to predict an atom's shell count without drawing its electron configuration. The presenter uses hydrogen, oxygen, aluminum, and calcium to illustrate the relationship between an element's period number and its electron shell count. Groups, or columns, are then explained as a means of organizing elements with similar chemical properties. The paragraph further explores the significance of valency and how it relates to an element's group, using the electronic configurations of group 1 and group 2 elements to show how the number of valence electrons determines an element's chemical properties and valency.
🎨 Group Numbering and Element Categories
The video script explains the shift from the old to the new group numbering scheme in the periodic table, highlighting the ease of the new system over the old one that used Roman numerals and letters. It provides a trick to remember the old scheme by referencing calcium, element number 20, and its corresponding group in the old system. The paragraph also introduces the color-coding of the periodic table to differentiate between metals, non-metals, metalloids, and noble gases. Special names for certain groups, such as alkali metals, alkaline earth metals, and noble gases, are discussed, along with the unique characteristics that warrant these group-specific names.
🌈 The Full Periodic Table and Its Merits
The final paragraph discusses the full periodic table, including periods six and seven, and the placement of elements like the lanthanide and actinide series. It emphasizes the merits of the modern periodic table, which corrects anomalies present in Mendeleev's table by being based on atomic number rather than atomic mass. The script addresses how the modern table accommodates isotopes and resolves discrepancies in element positioning, such as with cobalt and nickel. Hydrogen's unique position and properties are also noted. The presenter concludes by encouraging practice with the periodic table and to look forward to a subsequent video covering trends and property changes within the table. The video ends with a prompt to subscribe to the channel, follow on social media, and visit the website for further learning resources.
Mindmap
Keywords
💡Periodic Table
💡Atomic Number
💡Electron Configuration
💡Valency
💡Alkali Metals
💡Alkaline Earth Metals
💡Halogens
💡Noble Gases
💡Lanthanide and Actinide Series
💡Periodic Law
💡Isotopes
Highlights
The video introduces the modern periodic table by starting with a blank table and filling it up as the video progresses.
Focus is on the first 20 elements, covering only the first four rows, referred to as a 'mini periodic table'.
Atomic numbers, defined as the number of protons in an element's nucleus, are the basis for the modern periodic table, contrasting with Mendeleev's table based on atomic mass.
Elements are filled in the table sequentially by increasing atomic numbers, reflecting the addition of one proton in the nucleus for each element.
The video explains that the atomic number is more fundamental than atomic mass, correlating more closely with an element's chemical properties.
The significance of periods in the periodic table is that they represent the number of electron shells or orbits in an atom.
The video uses examples to show how the period number correlates with the number of electron shells for elements like hydrogen, oxygen, and aluminum.
Elements with similar chemical properties are grouped together in the periodic table, based on their electronic configurations.
The video discusses the valency and chemical similarity of elements within the same group, such as alkali metals (Group 1) and alkaline earth metals (Group 2).
The electronic configuration of elements in a group is analyzed to explain why they share the same valency and chemical properties.
The video provides an easy trick to remember the mapping between the new and old IUPAC group numbering schemes.
Different colors in the periodic table represent various categories of elements, such as metals, non-metals, metalloids, and noble gases.
Special names are given to certain groups, like the alkali metals, alkaline earth metals, boron family, carbon family, and noble gases.
Elements 57 to 71 (lanthanides) and 89 to 103 (actinides) are placed separately below the main table to compact the periodic table.
The modern periodic table corrects anomalies present in Mendeleev's table by basing its structure on atomic number rather than atomic mass.
Isotopes, which have the same atomic number but different mass numbers, fit neatly into the modern periodic table.
The video corrects the positions of certain elements like cobalt and nickel, which were exceptions in Mendeleev's table, based on atomic number.
Hydrogen is given special treatment in the periodic table due to its unique properties that are similar to both Group 1 and Group 17 elements.
The video encourages practice by drawing the mini periodic table and learning the first 20 elements to master the subject.
Transcripts
Browse More Related Video

Periodic Table of Elements Song
The Periodic Table of the Elements in Chemistry - [1-2-12]

3.2 Introduction to the Periodic Table | High School Chemistry

The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy

What is the Periodic Table? How are Elements Organized?

BTEC Applied Science: Unit 1 Chemistry The Periodic Table
5.0 / 5 (0 votes)
Thanks for rating: