3.2 Introduction to the Periodic Table | High School Chemistry
TLDRThis lesson introduces the periodic table, highlighting its organization by atomic numbers and the prediction of undiscovered elements by Mendeleev. It emphasizes the importance of memorizing the first 30 element symbols and explains the significance of groups and periods, including alkali metals, alkaline earth metals, halogens, and noble gases. The video also touches on the transition metals, metalloids, and the less commonly studied lanthanides and actinides.
Takeaways
- 🌟 Dmitri Mendeleev is credited as the 'father of the periodic table', having created a methodical and predictive table of elements.
- 🔍 The periodic table is organized by atomic numbers, which correspond to the number of protons in an element's nucleus.
- 📈 Atomic mass generally increases as you move down the table, but the table is specifically organized by atomic number.
- 🔬 Elements are arranged in such a way that those in the same column (group) often exhibit similar chemical properties, hence the term 'periodic'.
- 🧪 Mendeleev's table predicted the existence of elements that had not yet been discovered, showcasing his method's predictive power.
- 📚 It's recommended to memorize at least the first 30 element symbols for a basic understanding of the periodic table.
- 🔑 The atomic number is usually written at the top of the element symbol, and the atomic mass is found below it.
- 🌈 The periodic table has distinct groups, including alkali metals, alkaline earth metals, halogens, and noble gases, each with unique properties.
- 🛑 The alkali metals (Group 1) are highly reactive with water, forming basic solutions and are characterized by their violent reactions.
- 🌌 The noble gases are known for their chemical inertness, with most of them not participating in chemical reactions.
- 🛤️ A 'red staircase' in the periodic table separates metals from non-metals, with metalloids (semi-metals) having properties of both.
- 🌐 The transition metals, found in the middle of the table, are known for forming brightly colored compounds and are of significant interest in chemistry.
Q & A
Who is considered the father of the periodic table?
-Dmitri Mendeleev is considered the father of the periodic table.
What is the primary organization principle of the periodic table?
-The primary organization principle of the periodic table is atomic number, which corresponds to the number of protons in an element.
Why is the periodic table called 'periodic'?
-The periodic table is called 'periodic' because elements in the same column (group) often have similar chemical properties, and these properties repeat periodically across the table.
What are the names of the first two groups in the periodic table?
-The first two groups in the periodic table are the alkali metals and the alkaline earth metals.
What is the significance of the atomic number in the periodic table?
-The atomic number is significant because it determines the element's position in the periodic table and is equal to the number of protons in the nucleus of an atom.
What are the names of the last two groups mentioned in the script?
-The last two groups mentioned in the script are the halogens and the noble gases.
What are the characteristics of the noble gases?
-The noble gases are characterized by being chemically inert, meaning they are unreactive and do not easily form chemical bonds with other elements.
What is the term used to describe elements that have properties of both metals and non-metals?
-Elements that have properties of both metals and non-metals are called metalloids.
Why are the alkali metals reactive with water?
-The alkali metals are reactive with water because they form highly basic solutions when they react, causing the pH to rise significantly above seven.
What are the transition metals and why are they significant?
-The transition metals are elements found in the middle of the periodic table from scandium to zinc. They are significant because they often form brightly colored compounds and are widely used in various applications.
Outlines
🌐 Introduction to the Periodic Table
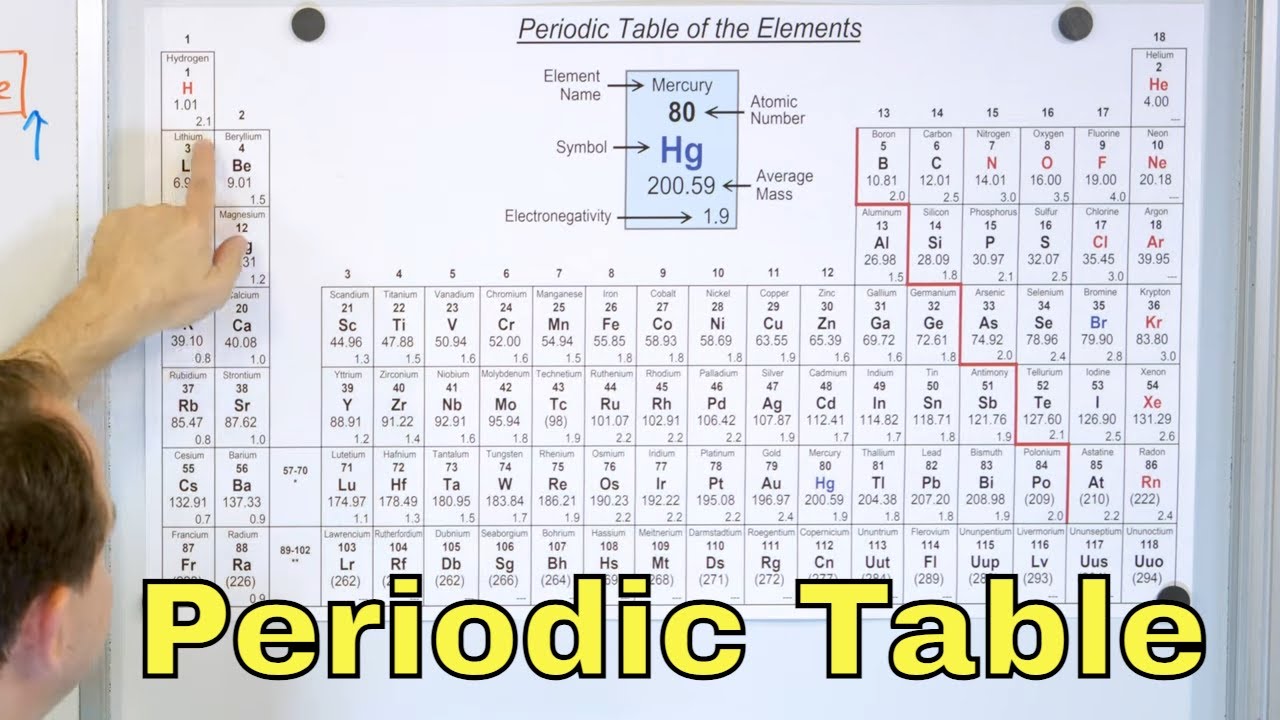
This paragraph introduces the periodic table, highlighting its significance and history. Mendeleev is credited with the creation of the table, which has evolved to include elements up to atomic number 118. The table is organized by atomic number, which corresponds to the number of protons, and generally reflects increasing atomic mass. Mendeleev's methodical approach allowed him to predict the existence of undiscovered elements. The paragraph emphasizes the importance of atomic numbers and the periodic nature of the table, where elements in the same column (groups) exhibit similar chemical properties. It also mentions the need to memorize the first 30 element symbols and discusses the concept of groups and periods in the table.
🔍 Understanding the Groups and Periods in the Periodic Table
This paragraph delves deeper into the structure of the periodic table, explaining the significance of groups and periods. It discusses the alkali metals (Group 1) and alkaline earth metals (Group 2), noting their reactivity with water and their tendency to form basic solutions. The halogens and noble gases are also introduced as key groups, with the former being highly reactive and the latter being chemically inert. The paragraph further explains the division of the table into metals, non-metals, and metalloids, with a focus on the transition metals in the middle of the table. It also briefly mentions the lanthanides and actinides, though their study is not considered essential at this level. The importance of recognizing the transition metals is emphasized, and the paragraph concludes with a call to action for viewers to engage with the provided study guide and premium course for further learning.
Mindmap
Keywords
💡Periodic Table
💡Atomic Number
💡Mendeleev
💡Atomic Mass
💡Element Symbol
💡Groups
💡Periods
💡Noble Gases
💡Metalloids
💡Alkali Metals
💡Transition Metals
Highlights
Introduction to the periodic table in the context of atomic theory and structure.
Mendeleev's role as the 'father' of the periodic table and his methodical approach to its creation.
The periodic table's expansion beyond Mendeleev's time, including elements with atomic numbers up to 118.
The periodic table's organization by atomic numbers, which correspond to the number of protons.
Importance of memorizing the first 30 element symbols for understanding the periodic table.
Explanation of the periodic table's name, derived from the periodic occurrence of elements with similar chemical properties.
The grouping of elements with similar chemical properties in the same column, known as groups.
The rows of the periodic table are called periods, further organizing the elements.
Characteristics of noble gases as chemically inert elements.
The periodic table's division into metals, non-metals, and metalloids.
Identification of alkali metals as group one elements and their basic properties.
Alkaline earth metals as group two elements and their reactivity with water.
Halogens as a group of elements including fluorine, chlorine, bromine, and iodine.
Noble gases as a group characterized by their lack of chemical reactivity.
The significance of transition metals in the middle of the periodic table, known for their colorful compounds.
The lesser-known groups of lanthanides and actinides, typically not studied in depth outside of advanced chemistry courses.
The importance of recognizing the transition metals in the periodic table.
Encouragement to engage with the study guide and practice materials for a deeper understanding of the periodic table.
Transcripts
Browse More Related Video

Periodic Table of Elements - Element Classes

Periodic Table of Elements Song
The Periodic Table of the Elements in Chemistry - [1-2-12]

Modern Periodic Table

The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy

Periodic Table of Elements Explained - Metals, Nonmetals, Valence Electrons, Charges
5.0 / 5 (0 votes)
Thanks for rating: