BTEC Applied Science: Unit 1 Chemistry The Periodic Table
TLDRThe video script introduces the periodic table, emphasizing the importance of understanding its structure and the elements' properties. It explains that there are 92 naturally occurring elements arranged by atomic mass, with about three-quarters being metals. The script highlights the significance of the atomic number and relative atomic mass, and how these relate to the elements' positions in the periodic table. It also discusses the concept of ionization energy and electron affinity, noting trends across periods and down groups. The video encourages learning the first 20 elements and understanding the s, p, and d blocks in relation to subshells and electrons. It concludes with a recommendation to watch a classic, humorous YouTube video for further chemistry insights.
Takeaways
- 📈 There are approximately 92 naturally occurring elements on the periodic table, which are ordered by their atomic mass.
- 🏰 Elements are categorized into groups and periods based on their similar properties, hence the term 'periodic table'.
- 🔢 The big number next to an element symbol is the relative atomic mass, while the small number is the atomic number, representing the number of protons in the element.
- 🎯 Metals make up about three-quarters of the elements on the periodic table.
- 🔶 The s, p, d, and f blocks on the periodic table correspond to different subshells and are related to the arrangement of electrons in atoms.
- 💥 First ionization energy refers to the minimum energy required to remove an electron from a neutral atom.
- 📊 As you move from left to right across a period, the first ionization energy increases due to the increasing nuclear charge attracting electrons more strongly.
- 📉 Moving down a group, the first ionization energy decreases because electrons are further from the nucleus, resulting in a weaker attractive force.
- 🌐 Non-metals have a higher electron affinity, meaning they tend to gain electrons and release energy when doing so.
- 🔝 Elements in group 17 (halogens) such as fluorine, chlorine, bromine, and iodine have the highest electron affinity among the non-metals.
- 🎓 Learning the first 20 elements and their properties is a fundamental and useful foundation in chemistry.
Q & A
How many naturally occurring elements are there in the periodic table?
-There are 92 naturally occurring elements in the periodic table.
What is the basis for the order of elements in the periodic table?
-Elements in the periodic table are ordered by their atomic mass, starting with hydrogen, which is the lightest, and increasing in mass as you move across the table.
What is the significance of the numbers next to each element in the periodic table?
-The big number next to each element represents the relative atomic mass, indicating how heavy the element is. The small number is the atomic number, which corresponds to the number of protons in the element's nucleus and its position in the periodic table.
What does the term 'periodic' in the periodic table refer to?
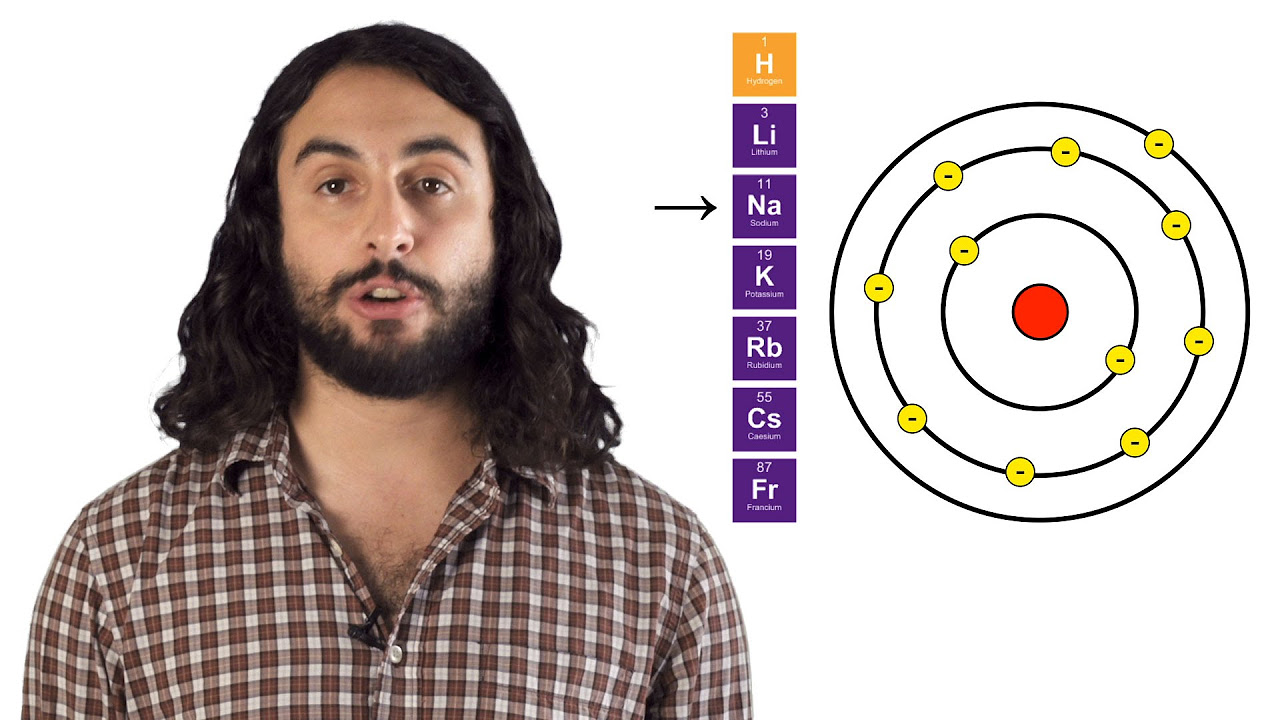
-The term 'periodic' refers to the periodic appearance of chemical properties among elements in the table. Elements in the same group have similar properties because they share the same number of electrons in their outermost shell.
What are the s, d, and f blocks in the periodic table, and how are they related to subshells?
-The s, d, and f blocks are sections of the periodic table that group elements with similar electron configurations in their subshells. These blocks are named after the types of subshells (s, d, f) that are being filled with electrons as you move through the table.
What is ionization energy, and how does it change as you move across a period and down a group in the periodic table?
-Ionization energy is the energy required to remove an electron from an atom. As you move across a period, the ionization energy increases because the nucleus has more protons, attracting the electrons more strongly. As you move down a group, the ionization energy decreases because the outer electrons are further from the nucleus and less tightly held.
Which elements have a high electron affinity?
-Non-metals, particularly those in group 17 (halogens like fluorine, chlorine, bromine, and iodine), have a high electron affinity because they are more likely to gain an electron, releasing the most energy in the process.
Why do some atoms want to lose an electron while others want to gain one?
-Atoms with one or two electrons in their outer shell tend to lose those electrons to achieve a stable, full outer shell. Conversely, atoms with nearly a full outer shell tend to gain electrons to complete that shell, resulting in a stable configuration.
What is the significance of learning the first 20 elements of the periodic table?
-Learning the first 20 elements is beneficial because it provides a foundational understanding of the periodic table and its patterns. This knowledge is practical and can be applied in various chemistry-related fields and studies.
How does the 3D graphic in the video help to understand ionization energy trends?
-The 3D graphic visually represents the trends in ionization energy across periods and down groups, making it easier to see how the energy changes in relation to an element's position in the periodic table.
What is the relationship between an element's electron affinity and its position in the periodic table?
-Elements with high electron affinity are typically non-metals located in group 17 of the periodic table. These elements are more likely to gain an electron and release a significant amount of energy when they do so.
Outlines
📚 Introduction to the Periodic Table
This paragraph introduces the concept of the periodic table, highlighting its initial intimidating appearance due to the vast number of elements. It emphasizes the importance of understanding the basics, such as the number of naturally occurring elements (92), the reason behind their ordering (by atomic mass), the prevalence of metals (about three-quarters), and the significance of the grouping and numbering system. The explanation includes the meaning of the big and small numbers next to each element, representing the relative atomic mass and atomic number, respectively. The speaker shares a personal anecdote about the usefulness of memorizing the first 20 elements and introduces the concept of the s, d, and p blocks, as well as the transition metals and their relation to subshells and electrons. The concept of ionization energy is also introduced, explaining its significance and how it varies across periods and down groups in the periodic table.
🔬 Trends in Ionization and Electron Affinity
This paragraph delves into the trends of ionization and electron affinity within the periodic table. It explains how the ionization energy generally increases as you move from left to right across a period and decreases as you move down a group, attributing these trends to the increasing nuclear charge and the distance of electrons from the nucleus. The paragraph also discusses electron affinity, focusing on non-metals and their tendency to gain electrons, with group 17 elements (halogens) having the highest electron affinity. A visual 3D graphic is mentioned to illustrate these trends, and a recommendation is made to watch a specific YouTube video for further understanding of these concepts.
Mindmap
Keywords
💡Periodic Table
💡Atomic Mass
💡Atomic Number
💡Metals
💡Groups
💡Periods
💡Ionization Energy
💡Electron Affinity
💡S-Block, P-Block, D-Block, and F-Block
💡First Ionization Energy
💡Electron Shells
Highlights
There are 92 naturally occurring elements in the periodic table.
Elements are ordered by their atomic mass, starting with the lightest hydrogen.
Approximately three quarters of the elements are metals.
Elements are grouped based on similar properties, which appear periodically.
The big number next to an element is its relative atomic mass, and the small number is the atomic number.
Hydrogen is unique and placed on its own due to its distinct properties.
The first 20 elements are crucial to learn, especially for beginners in chemistry.
The s, p, d, and f blocks in the periodic table are related to electron subshells.
First ionization energy is the minimum energy required to remove an electron from an atom.
Atoms with one or two electrons tend to lose them easily, while those with nearly full outer shells want to gain electrons.
As you move across a period, the first ionization energy increases due to the increasing number of protons attracting the electron.
Elements in period three have smaller first ionization energies than those in period two because their electrons are further from the nucleus.
Electron affinity is the energy released when an atom gains an electron, which is more relevant for non-metals.
Group 17 elements (halogens) have the highest electron affinity, releasing the most energy when gaining an electron.
Two key trends in the periodic table: ionization energy increases across a period and decreases down a group.
The video mentioned is praised for its clever and humorous explanation of chemistry concepts, particularly related to the periodic table.
Revision questions are provided for viewers to test their understanding of the concepts discussed in the video.
Transcripts
Browse More Related Video
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity

Modern Periodic Table

Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character

Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series

Other Periodic Table Trends

How to Write the Electron Configuration for an Element in Each Block
5.0 / 5 (0 votes)
Thanks for rating: