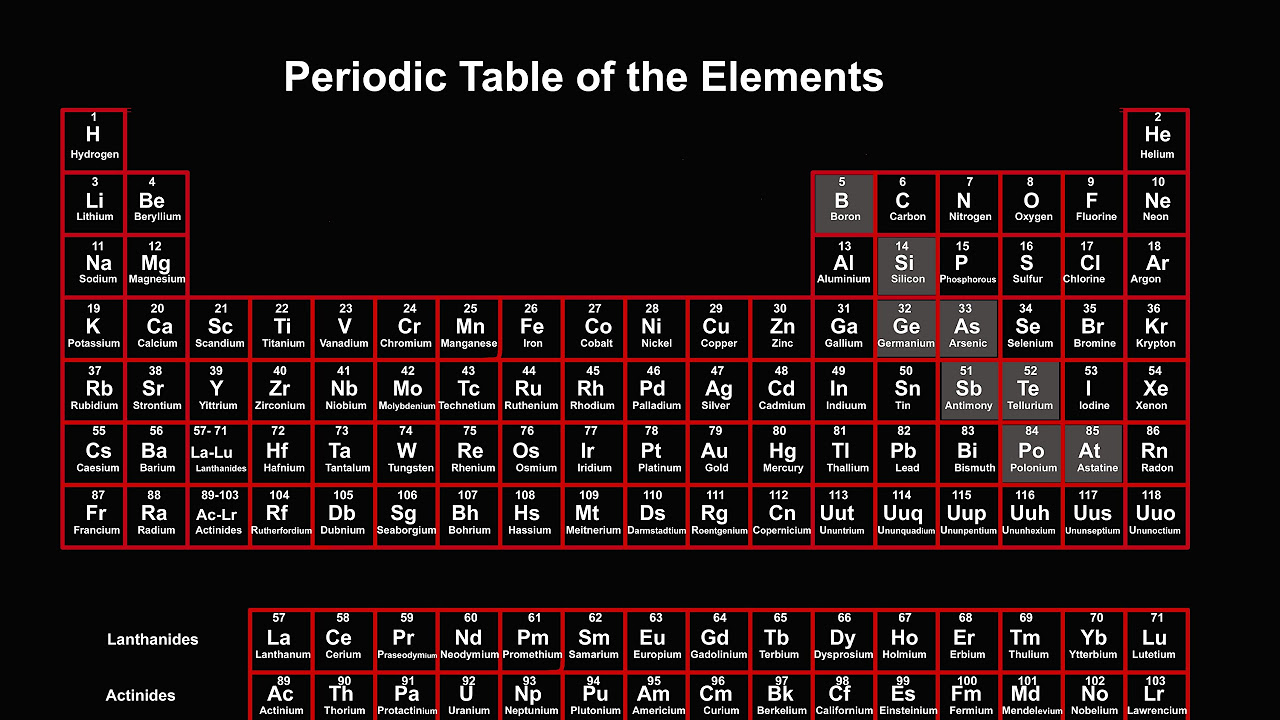
The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy
TLDRThe video script offers an insightful exploration into the periodic table, detailing how elements are categorized into groups and periods. It explains the vertical columns as groups, numbered from 1 to 18, with an alternative numbering system (1A to 8A) that aids in understanding valence electrons. The script delves into specific groups, such as alkali metals (group 1 or 1A) and alkaline earth metals (group 2 or 2A), highlighting their reactivity and typical states in nature. It contrasts metals, which are malleable, ductile, and good conductors, with nonmetals, which are brittle and poor conductors. The halogens (group 7A or 17) and noble gases (group 8A or 18) are introduced as examples of very reactive nonmetals and unreactive colorless gases, respectively. The video also touches on metalloids, which possess properties between metals and nonmetals, using silicon as a notable example. The periodic table's layout is depicted, with metals on the left, nonmetals on the right, and a zigzag line dividing them. The summary sets the stage for a deeper discussion on electronic structure and transition metals in a subsequent video.
Takeaways
- 📊 The periodic table is organized into groups (vertical columns) and periods (horizontal rows).
- 🔢 Groups are numbered from 1 to 18, with an alternative notation of 1A to 8A, excluding groups 3-12.
- 🌟 Group 1 elements, known as alkali metals (e.g., lithium, sodium, potassium), are soft, silvery, and highly reactive.
- 💧 Alkali metals react with water and are not found in pure form in nature; they are combined with other elements.
- ⚛️ Hydrogen, although in group 1, is a nonmetal and does not share the same properties as alkali metals.
- 🌱 Alkaline earth metals, found in group 2, are less reactive than alkali metals but also not found in pure form in nature.
- 🔩 Groups 3 through 12 are all metals, which are generally solid at room temperature, malleable, ductile, and good conductors of heat and electricity.
- 🔺 Nonmetals are found on the right side of the periodic table and are poor conductors of heat and electricity, with some being brittle solids.
- ⚡ Halogens, located in group 7A or 17, are very reactive nonmetals known for their corrosive properties and colorful appearance.
- 🌌 Noble gases, in group 8A or 18, are colorless, unreactive gases, and are known for their stability.
- 🔶 Metalloids, found along the zigzag line dividing metals from nonmetals, have properties intermediate between the two.
- 💠 Silicon, a well-known metalloid, is a semiconductor and conducts electricity, though not as well as metals.
Q & A
What is the significance of the vertical columns on the periodic table?
-The vertical columns on the periodic table are called groups. Elements in the same group have similar chemical properties due to having the same number of valence electrons.
How are groups on the periodic table numbered in the first method?
-In the first method, groups are numbered from 1 to 18 sequentially, following the vertical columns from left to right.
What is the alternative numbering system for groups on the periodic table?
-The alternative numbering system uses letters A to denote groups, starting with group 1 as 1A and group 2 as 2A, and then continuing with 3A, 4A, etc., up to 8A, ignoring the groups 3 through 12.
What is a period on the periodic table?
-A period is a horizontal row on the periodic table, and elements in the same period have the same number of electron shells.
Why are alkali metals not found in their pure state in nature?
-Alkali metals are extremely reactive and readily react with other elements, which is why they are not found in their pure state in nature but rather in combination with other elements.
What sets hydrogen apart from other elements in group 1?
-Hydrogen is a nonmetal and is the exception in group 1, which primarily consists of alkali metals. It has different chemical properties compared to the other elements in the group.
What are the common characteristics of alkaline earth metals?
-Alkaline earth metals, found in group 2 or 2A, are reactive metals but not as reactive as alkali metals. They are not found in their pure state and are typically found in combination with other elements.
How do the properties of metals differ from nonmetals?
-Metals are typically solid at room temperature (except for mercury), malleable, ductile, and good conductors of heat and electricity. Nonmetals, on the other hand, are often brittle, poor conductors of heat and electricity, and can be found in different states of matter.
What is the significance of the term 'halogen' for elements in group 7A or 17?
-The term 'halogen' means 'salt former' and refers to very reactive nonmetals in group 7A or 17, such as fluorine, chlorine, and bromine. They are often colorful and corrosive.
Why are noble gases considered unreactive?
-Noble gases, found in group 8A or 18, are considered unreactive because they have a full complement of valence electrons, making them stable and less likely to form chemical bonds.
What is the dividing line between metals and nonmetals on the periodic table?
-The dividing line between metals and nonmetals on the periodic table is a zigzag line that separates the left side, which is primarily metals, from the right side, which is primarily nonmetals.
What are metalloids and where are they located on the periodic table?
-Metalloids are elements with properties intermediate between metals and nonmetals. They are found along the zigzag line that separates metals from nonmetals and include elements like boron, silicon, and germanium.
Outlines
🔍 Introduction to the Periodic Table and Element Groups
This paragraph introduces the viewer to the periodic table, emphasizing the classification of elements into groups based on their vertical columns. The narrator explains two numbering systems for groups: the traditional one through 18, and the alternative one with letters A (1A to 8A) which is more relevant for understanding valence electrons. The concept of periods as horizontal rows is also introduced, with examples of elements in periods 1 and 2. The paragraph concludes by mentioning that not all elements will be discussed due to space constraints and focuses on metals, specifically alkali metals found in group 1 or 1A, which are soft, silvery, and highly reactive, sharing similar chemical properties.
🌟 Properties of Metals, Nonmetals, and Metalloids
The second paragraph delves into the properties of metals, nonmetals, and metalloids. Metals, which are generally found on the left side of the periodic table, are characterized by being solid at room temperature (except for mercury), malleable, ductile, and good conductors of heat and electricity. Nonmetals, often found in various states of matter, are poor conductors of heat and electricity, and are typically brittle solids. The paragraph specifically discusses halogens (group 7A or 17) as very reactive nonmetals, known for their corrosive nature and colorful appearance, and noble gases (group 8A or 18), which are colorless, unreactive gases. The dividing line between metals and nonmetals is illustrated as a zigzag line, with elements on this line, known as metalloids, exhibiting properties intermediate between metals and nonmetals. Metalloids such as silicon, which is a semiconductor, are highlighted for their utility due to their unique properties. The paragraph ends by briefly mentioning that more details on electronic structure and transition metals will be covered in the next video.
Mindmap
Keywords
💡Periodic Table
💡Groups
💡Periods
💡Alkali Metals
💡Hydrogen
💡Alkaline Earth Metals
💡Metals
💡Nonmetals
💡Halogens
💡Noble Gases
💡Metalloids
Highlights
The periodic table is organized into groups, which are the vertical columns, and periods, which are the horizontal rows.
Groups are numbered from 1 to 18, with an alternative system using A (1A to 8A) for groups 1 and 2, and ignoring groups 3 through 12.
Elements within the same group have similar chemical properties, which is useful for predicting their reactivity.
Alkali metals, found in group 1 or 1A, are soft, silvery, and extremely reactive, never found in pure state in nature.
Hydrogen, despite being in group 1, is a nonmetal and does not share the same properties as alkali metals.
Alkaline earth metals, located in group 2 or 2A, are less reactive than alkali metals but still not found in pure state in nature.
Metals are generally solid at room temperature, malleable, ductile, and good conductors of heat and electricity.
Nonmetals are often brittle, poor conductors of heat and electricity, and can be found in different states of matter.
Halogens, found in group 7A or 17, are very reactive nonmetals known for being colorful and corrosive.
Noble gases, in group 8A or 18, are colorless, unreactive gases with complete electron shells.
Metals are typically found on the left side of the periodic table, while nonmetals are on the right side.
The dividing line between metals and nonmetals is a zigzag line, with elements on this line known as metalloids.
Metalloids, such as silicon, have properties intermediate between metals and nonmetals and are used as semiconductors.
Silicon is a well-known metalloid used in the electronics industry due to its semiconducting properties.
Elements considered metalloids can vary slightly between different sources, but generally include boron, silicon, germanium, arsenic, antimony, and tellurium.
The periodic table's organization helps predict how elements will react and their general properties based on their group and period.
Further discussion on electronic structure and the definition of transition metals will be covered in the next video.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: