Chem1, Vol1 Sect16 Hydrate Compounds
TLDRThe provided transcript is a chemistry tutorial that demystifies the concept of hydrate compounds. The instructor emphasizes that while these compounds may sound complex, they are essentially ionic compounds with a fixed number of water molecules integrated into their lattice structure. The tutorial explains that hydrate compounds are commonly found in nature, often formed when a solution of dissolved ions evaporates, leaving behind a crystalline structure with water molecules trapped within. The naming convention for these compounds is also covered, illustrating how to denote the number of water molecules with prefixes such as 'hexa' for six and 'di' for two. The instructor reassures students that understanding the rules of chemistry makes tackling even the most intimidating sounding compounds, like lithium perchlorate trihydrate, quite manageable. The summary aims to clarify that chemistry is governed by rules, which once understood, allow for a deeper appreciation and mastery of the subject.
Takeaways
- 💧 **Hydrate Compounds Involve Water**: A hydrate compound is an ionic compound with a fixed number of water molecules incorporated into its crystal lattice.
- 🧬 **Formation of Hydrates**: Hydrate compounds are commonly formed when a solution of dissolved ions evaporates, leaving behind a crystalline structure with water molecules trapped within.
- 🌿 **Natural Occurrence**: Hydrates are often found in nature, such as in the formation of stalagmites and stalactites in caves, where water evaporates from dissolved minerals.
- 📏 **Crystal Lattice Structure**: The water molecules in a hydrate compound are not part of the ionic compound itself but are simply trapped within the rigid lattice structure.
- 📚 **Naming Hydrates**: Hydrate compounds are named by first identifying the ionic compound and then indicating the number of water molecules, using prefixes like 'hexa' for six and 'di' for two.
- 🔍 **Microscopic View**: Under a microscope, one can observe the water molecules within the lattice structure of an ionic compound, showing that they are not chemically bonded but physically trapped.
- 🚫 **No Need to Memorize**: There's no need to memorize which ionic compounds form hydrates; instead, understand how to recognize and name them when encountered.
- ✍️ **Writing Formulas**: The chemical formula of a hydrate compound is written by first writing the ionic compound and then adding the water molecules (H2O) with the appropriate prefix indicating the quantity.
- 🔢 **Understanding Prefixes**: Prefixes like 'tri' for three, 'tetra' for four, 'penta' for five, and 'hepta' for seven are used to denote the number of water molecules in the hydrate.
- 🧪 **Practical Application**: Knowledge of hydrate compounds is important for understanding how to name and write the formulas for these compounds, which is a fundamental skill in chemistry.
- 📈 **Building on Basics**: The process of naming and formulating hydrates builds on the basic principles of naming ionic compounds and polyatomic ions, emphasizing the importance of understanding these rules.
Q & A
What is the basic definition of a hydrate compound?
-A hydrate compound is an ionic compound that contains a fixed number of water molecules incorporated within its crystal lattice structure.
How are hydrate compounds typically formed in nature?
-Hydrated compounds are usually formed when a solution, such as one found in a pond or puddle, evaporates over time, leaving behind a crystalline structure with water molecules trapped within the lattice.
What does the term 'hexahydrate' signify in the chemical name?
-The term 'hexahydrate' signifies that there are six water molecules tied up within the lattice structure of the ionic compound.
How is the chemical formula for a hydrate compound written?
-The chemical formula for a hydrate compound is written by first writing the ionic compound formula, followed by a dot and the number of water molecules (e.g., CaCl2·6H2O for calcium chloride hexahydrate).
What is the prefix 'dihydrate' indicating in a chemical name?
-The prefix 'dihydrate' indicates that there are two water molecules associated with each formula unit of the ionic compound.
How do you name a hydrate compound with lithium perchlorate and three water molecules?
-The compound would be named lithium perchlorate trihydrate, where 'tri' signifies three water molecules within the lattice structure.
What is the process of naming a hydrate compound in reverse?
-To name a hydrate compound in reverse, you start by identifying the ionic part of the compound, then determine the charges of the ions involved, crisscross the charges to get the formula, and finally, add the prefix that corresponds to the number of water molecules in the hydrate part.
Why are hydrate compounds not considered difficult to understand?
-Hydrate compounds are not considered difficult because their naming and understanding follow the same rules as regular ionic compounds, with the addition of a water molecule count to indicate the number of water molecules within the lattice.
What is the significance of understanding the rules of chemistry?
-Understanding the rules of chemistry allows one to make sense of even complex-sounding compounds and reactions, enabling the prediction and manipulation of chemical behavior in various applications.
What is the prefix used for five water molecules in a hydrate compound?
-The prefix used for five water molecules in a hydrate compound is 'penta', as in magnesium carbonate pentahydrate.
What is the charge that aluminum typically forms in its compounds?
-Aluminum typically forms a +3 charge in its compounds.
How many water molecules are indicated in the term 'heptahydrate'?
-The term 'heptahydrate' indicates that there are seven water molecules associated with the ionic compound.
Outlines
🌟 Understanding Hydrate Compounds
This paragraph introduces the concept of hydrate compounds, which are ionic compounds with water molecules incorporated into their lattice structure. The video explains that despite their intimidating name, these compounds are quite simple. They form naturally when a solution of ions evaporates, leaving behind a crystalline structure with water molecules trapped within. The script clarifies that water in hydrate compounds is not part of the ionic compound itself but is simply trapped in the lattice. The naming convention for these compounds is also discussed, highlighting the use of prefixes like 'hexa' for six and 'di' for two to indicate the number of water molecules.
📚 Naming and Formulating Hydrate Compounds
The second paragraph delves into the specifics of naming and writing the chemical formulas for hydrate compounds. It emphasizes that the process involves identifying the ionic part of the compound first, using standard nomenclature rules, and then indicating the number of water molecules present with appropriate prefixes (e.g., 'trihydrate' for three water molecules). Examples given include lithium perchlorate trihydrate, magnesium carbonate pentahydrate, and barium iodide dihydrate. The paragraph reassures that memorizing which compounds form hydrates is not necessary; instead, understanding the naming and formulating process is key.
🔬 Simplifying Chemistry: Rules and Reactions
The final paragraph reinforces the idea that chemistry is governed by rules, which once understood, make the subject more accessible. It suggests that the initial complexity of chemistry lessens as one learns the rules, allowing for the comprehension of even seemingly complicated compounds. The script hints at upcoming topics in the chemistry course, such as chemical reactions, limiting reactants, and product calculations. The presenter, Jason, encourages students to review the material and assures them that a solid understanding of these principles will lead to success in their chemistry studies and exams.
Mindmap
Keywords
💡Hydrate Compounds
💡Ionic Compound
💡Crystal Lattice
💡Chemical Nomenclature
💡Evaporation
💡Stalagmites and Stalactites
💡Polyatomic Ions
💡Charge Balance
💡Hexahydrate, Dihydrate, Trihydrate
💡Cation and Anion
💡Chemical Formula
Highlights
Hydrate compounds are ionic compounds with a fixed number of water molecules in the lattice structure.
The term 'hydrate' refers to the inclusion of water molecules within the compound, similar to hydrating a body.
Hydrate compounds form naturally when a solution with dissolved ions evaporates, leaving behind crystalline structures like stalagmites and stalactites.
The water in hydrate compounds is not part of the ionic compound but is trapped within the lattice structure.
Hydrate compounds are commonly found in chemical supply houses for experimental use.
The naming convention for hydrate compounds involves the prefix indicating the number of water molecules, followed by 'hydrate'.
For example, 'hexahydrate' means six water molecules are present in the compound.
The process of naming hydrate compounds follows the same rules as naming ionic compounds, with the addition of water molecule count.
The chemical formula of a hydrate compound is written by first writing the ionic compound and then indicating the number of water molecules.
An example of a hydrate compound is calcium chloride hexahydrate, which has six water molecules in its lattice.
Understanding the rules of chemistry makes complex-sounding compounds like lithium perchlorate trihydrate more approachable.
The 'trihydrate' in lithium perchlorate trihydrate indicates that there are three water molecules within the lattice structure.
Magnesium carbonate pentahydrate is named by identifying the ionic compound and then adding 'pentahydrate' for five water molecules.
Zinc sulfate heptahydrate contains seven water molecules, as indicated by the prefix 'hepta'.
The ability to name and write the formula of hydrate compounds is an essential skill in chemistry, focusing on understanding rather than memorization.
Hydrate compounds demonstrate the rule-following nature of chemistry, making complex concepts more manageable once the rules are understood.
The course aims to help students appreciate chemistry by understanding its rules and applying them to complex concepts like hydrate compounds.
The next steps in the course will involve chemical reactions, calculating product quantities, and identifying limiting reactants.
Transcripts
Browse More Related Video

Lesson 16 - Hydrate Compounds in Chemistry

Lesson 13 - Ions And Ionic Charge (Chemistry Tutor)

What Happens when Stuff Dissolves?

Intro to Elements, Compounds, & the Periodic Table - [1-1-3]

Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]
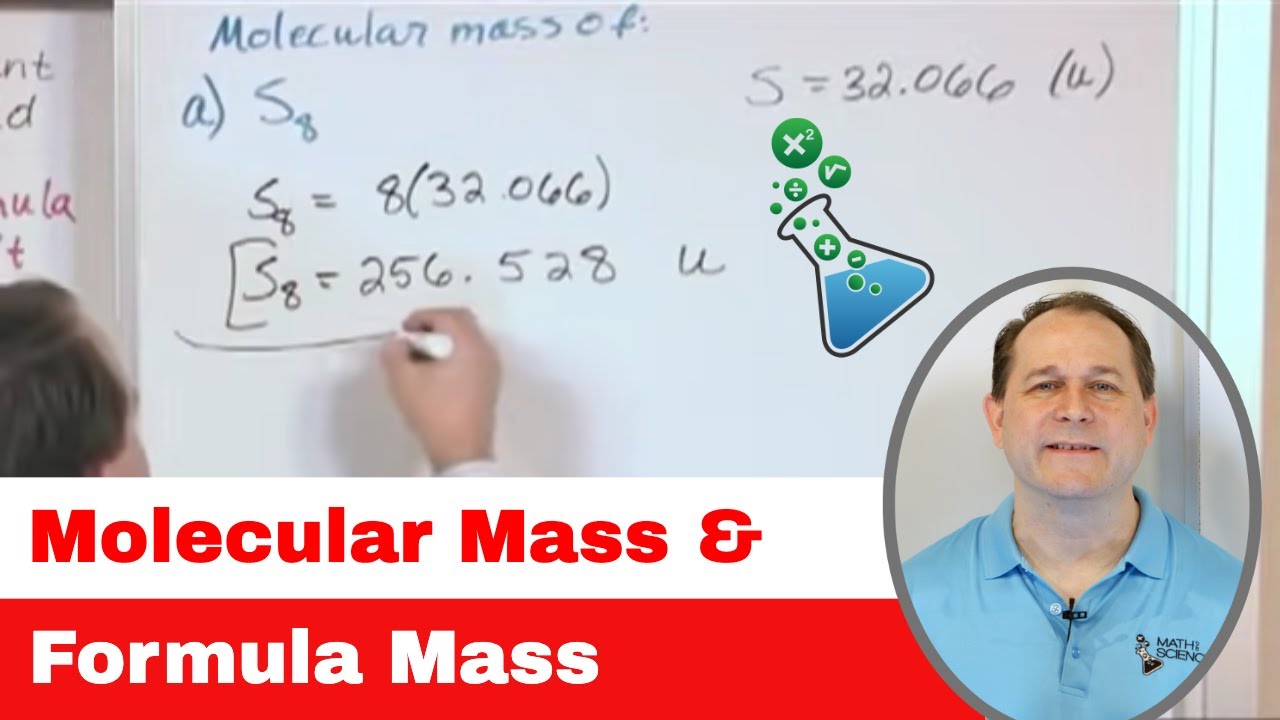
01 - Molecular Mass And Formula Mass - Learn the Formula Unit, Molecular Formula & Formula Mass
5.0 / 5 (0 votes)
Thanks for rating: