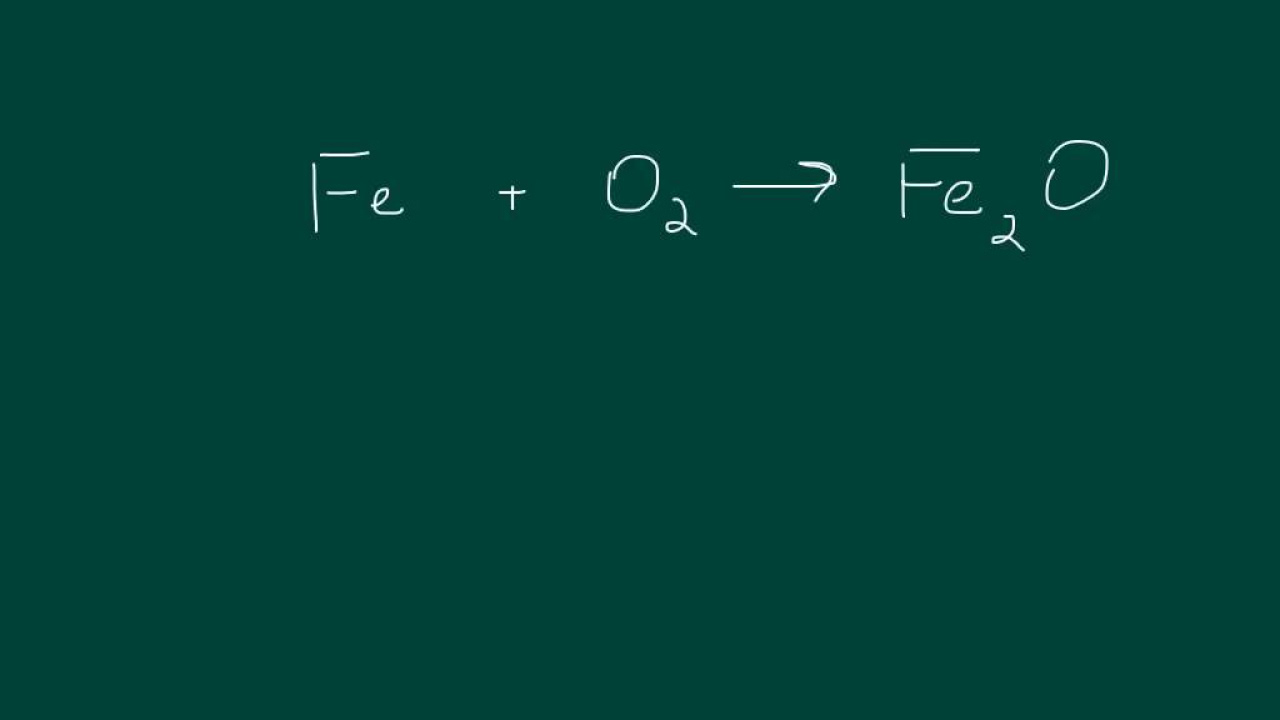Balancing Chemical Equations Step by Step Practice Problems | How to Pass Chemistry
TLDRThe video script offers a comprehensive guide on balancing chemical equations, a process that may initially seem daunting but is broken down into manageable steps by the presenter, Melissa Maribel. She emphasizes the importance of balancing reactants and products to ensure an equal number of each element on both sides of the equation. The video suggests starting with elements other than hydrogen and oxygen, which are addressed last due to their tendency to balance out easily. Melissa provides a step-by-step approach, illustrating how to count elements, distribute coefficients without breaking compounds, and recount elements after each adjustment. She also advises on handling polyatomic groups like OH and the strategy of balancing elements in a specific order: chlorine, sodium, and then OH groups. The script concludes with an encouragement to practice, as it is key to mastering chemistry, and an invitation to join live tutoring sessions for further assistance. This summary encapsulates the essence of the video, highlighting its educational value and the presenter's engaging teaching style.
Takeaways
- 🧪 Balancing chemical equations requires ensuring the same number of each element on both sides of the equation.
- 📝 Start by balancing elements other than hydrogen and oxygen, which are typically addressed last.
- 🔬 Keep the order of elements consistent on both sides of the equation to simplify the balancing process.
- 🧐 Do not separate polyatomic groups like OH when they appear on both sides of the equation.
- 📊 Count the number of each element in the reactants and products before starting to balance.
- ➡️ Begin balancing with chlorine or other non-hydrogen and non-oxygen elements that have a difference in quantity between reactants and products.
- 🚫 Avoid placing coefficients inside a compound as it would imply breaking the compound apart.
- ✅ After balancing one element, recount all elements to ensure the equation remains unaltered for other elements.
- 🌟 Address hydrogen second to last and oxygen last, as they often balance out more easily at the end of the process.
- 🔄 When balancing, multiply the subscript numbers by the balancing coefficient to find the total number of atoms.
- ⚖️ The final step is to ensure that all elements, including hydrogen and oxygen, are balanced equally on both sides of the equation.
- 📈 Practice is essential for mastering the skill of balancing chemical equations, and additional resources like practice problems and tutoring sessions can be beneficial.
Q & A
What is the primary goal when balancing chemical equations?
-The primary goal is to ensure that the number of atoms for each element is the same on both the reactants and products sides of the equation.
Why should we start balancing chemical equations with elements other than hydrogen and oxygen?
-Starting with elements other than hydrogen and oxygen simplifies the process because hydrogen and oxygen are often more complex to balance due to their frequent presence in chemical compounds.
What is the significance of not separating polyatomic groups like OH when balancing chemical equations?
-Keeping polyatomic groups intact ensures that the structure and properties of the compound are maintained, which is crucial for accurate balancing.
How does the subscript next to an element symbol in a chemical equation affect the balancing process?
-The subscript indicates the number of atoms of that element within a single molecule or ion. It directly affects the balancing process by determining the total count of atoms that need to be matched on both sides of the equation.
Why is it incorrect to place a coefficient in the center of a compound symbol?
-Placing a coefficient in the center of a compound symbol would imply breaking the compound into separate elements, which changes its chemical identity and is not allowed in balancing equations.
What happens when you add a coefficient in front of a compound in a chemical equation?
-Adding a coefficient in front of a compound multiplies the entire compound by that number, affecting the count of all elements within the compound.
How does the process of recounting help in balancing chemical equations?
-Recounting the number of atoms after adding coefficients ensures that the new totals are accurately reflected, helping to verify that the equation is balanced.
Why is it recommended to balance hydrogen second to last and oxygen last when balancing chemical equations?
-Hydrogen and oxygen often appear in many reactions and are involved in the formation of water, which is a common product. Balancing them last makes it easier to achieve a balanced equation.
What is the purpose of practice in mastering the skill of balancing chemical equations?
-Practice helps solidify the understanding of the balancing process and allows for the recognition of patterns and shortcuts in more complex equations.
What should one do if they have a specific question about balancing chemical equations?
-One should leave a comment below the video or join a live tutoring session to ask their question and get detailed help.
What is the role of 'likes' and 'subscriptions' in the context of the video on balancing chemical equations?
-'Likes' and 'subscriptions' support the content creator by promoting their videos and ensuring that they continue to produce educational content.
How does the video transcript help in understanding the process of balancing chemical equations?
-The transcript provides a detailed, step-by-step guide that can be revisited and studied at one's own pace, which is particularly useful for visualizing and understanding the process of balancing equations.
Outlines
🔬 Balancing Chemical Equations - An Introduction
Melissa Maribel introduces the concept of balancing chemical equations, emphasizing the importance of having the same number of reactants and products. She advises starting with elements other than hydrogen and oxygen and provides a step-by-step guide on how to balance an equation. She also highlights the importance of not separating polyatomic atoms like OH and to keep them intact for easier balancing. The process involves counting the number of each element on both sides of the equation and adjusting the coefficients to achieve balance, starting with chlorine and then balancing sodium and barium, leaving hydrogen and oxygen for last.
🧪 Advanced Balancing Techniques and Practice
The second paragraph delves into more advanced balancing techniques, reminding viewers to write out all elements in the same order on both sides of the equation. It focuses on balancing elements other than hydrogen and oxygen first. The example provided involves counting the number of carbon, hydrogen, and oxygen atoms on both sides of the equation. The process shown involves placing coefficients in front of compounds to balance carbon and then hydrogen, before finally balancing oxygen. The video script encourages practice as a key to understanding chemistry and offers additional resources, such as more practice problems and live tutoring sessions, for those who need further help. It concludes with an invitation for viewers to engage with the content by liking, subscribing, and commenting with questions.
Mindmap
Keywords
💡Chemical Equations
💡Balancing
💡Reactants
💡Products
💡Polyatomic Ions
💡Hydrogen and Oxygen
💡Subscripts
💡Coefficients
💡Stoichiometry
💡Law of Conservation of Mass
💡Practice Problems
Highlights
Balancing chemical equations requires ensuring the same number of reactants and products.
Begin balancing with elements other than hydrogen and oxygen.
Keep polyatomic atoms like OH intact on both sides of the equation for easier balancing.
Count the number of each element in reactants and products to identify imbalances.
Balance chlorine first since it has a discrepancy between reactants and products.
Adding a coefficient to a compound distributes to all elements within that compound.
Recount elements after adding coefficients to maintain balance.
Balance sodium (Na) by ensuring equal amounts on both sides of the equation.
Once other elements are balanced, address hydrogen second to last.
Balance hydrogen by adjusting coefficients to match the number of hydrogen atoms.
Oxygen is typically balanced last due to its frequent presence in compounds.
Multiply coefficients by subscript numbers to balance elements with subscripts.
Practice is key to understanding chemistry and balancing chemical equations.
There are additional practice problems and live tutoring sessions available for further assistance.
Engage with the community by leaving comments for specific questions or help needed.
The presenter emphasizes the importance of understanding the process rather than memorization.
The video provides a step-by-step guide to balance a specific chemical equation.
The presenter suggests starting with carbon when balancing more complex equations.
Recall and redistribute coefficients to balance all elements systematically.
Ensure all elements are balanced before considering the equation complete.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: