Inside Atoms: Electron Shells and Valence Electron
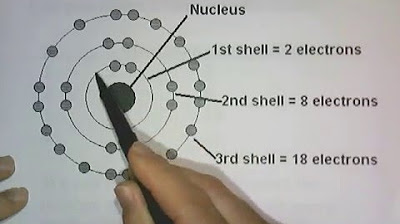
TLDRThis script delves into the intricate arrangement of electrons in atoms, drawing parallels with our solar system to illustrate the concept. It explains that electrons, like planets, occupy fixed energy levels or 'shells' around the nucleus, with each shell having a specific capacity for electrons. The first shell can hold up to 2 electrons, the second up to 8, and so on, with the arrangement following a set order based on energy levels. The script also introduces the concept of valence electrons, which are the outermost electrons crucial for chemical bonding. It concludes with a teaser about the periodic table's significance in chemistry and a future exploration of how valence electrons form stable compounds through bonding.
Takeaways
- 🌌 An atom is made up of a nucleus with neutrons and protons, and electrons that move around the nucleus in orbitals.
- 📊 Electrons occupy shells that are energy levels, with the first shell closest to the nucleus having the lowest energy level.
- 🔋 Each shell can hold a specific number of electrons, with the first shell holding a maximum of 2, the second 8, and so on.
- 🧲 Electron shells are filled in order of increasing energy levels, starting with the lowest energy level first.
- 💡 The electron configuration of an element can be represented in a diagram, showing how its electrons are arranged across different shells.
- ⚛️ Hydrogen, with an atomic number of 1, has a single electron in its first shell, which is also its valence electron.
- 🅰️ Carbon has an atomic number of 6, with 2 electrons in the first shell and 4 in the second, totaling its valence electrons.
- 🔆 Sulfur, with an atomic number of 16, has 2 electrons in the first shell, 8 in the second, and 6 in the third, making its valence electron count 6.
- 📊 Calcium, with an atomic number of 20, follows the configuration of 2, 8, 8, and 2, filling each shell to its maximum before moving to the next.
- 🔬 For elements with atomic numbers greater than 20, the filling pattern of the third shell and beyond differs, with the third shell able to hold up to 18 electrons.
- 🔬 Valence electrons are the outermost electrons in an atom's shell and play a crucial role in chemical bonding.
- 📚 The periodic table organizes all elements by increasing atomic (proton) numbers, with 7 periods and 18 groups, and is fundamental to chemistry.
Q & A
What are the main components of an atom?
-An atom consists of a nucleus, which contains neutrons and protons, and electrons that move around the nucleus in orbitals.
How do the shapes of electron orbitals vary?
-The shape of orbitals varies with the number of electrons present in the atom.
What is the analogy used to understand the arrangement of electrons in an atom?
-The arrangement of electrons is compared to our solar system, where electrons are like planets orbiting the sun.
How are electron shells related to energy levels?
-Each electron shell has a specific energy level, and electrons are fixed in their shells without colliding, similar to how planets are in fixed orbits.
In what order are electron shells filled?
-All electron shells are filled in order of increasing energy levels, starting with the lowest energy level closest to the nucleus.
What is the maximum number of electrons the first shell can hold?
-The first shell can hold at most 2 electrons.
How many electrons can the second shell hold at maximum?
-The second shell can hold a maximum of 8 electrons.
What is the term used to describe the outermost shell of an atom?
-The outermost shell of an atom is known as the valence shell.
What are valence electrons?
-Valence electrons are the electrons in the outer shell of an atom, also referred to as outer electrons.
How does the periodic table of elements organize elements?
-The periodic table organizes elements according to their increasing proton numbers, with 7 rows called periods and 18 columns called groups.
What is the significance of valence electrons in chemistry?
-Valence electrons play a crucial role in chemical bonding, as they join together to form stable compounds through a process called bonding.
What happens to the electron configuration when the atomic number is more than 20?
-When the atomic number exceeds 20, the filling pattern of the third shell and subsequent shells changes, with the third shell being able to fit up to 18 electrons.
Outlines
🌌 Electron Arrangement in Atoms
This paragraph explains the structure of an atom, which includes a nucleus with neutrons and protons and electrons that orbit the nucleus in areas known as orbitals. It uses the analogy of our solar system to describe how electrons occupy shells or energy levels around the nucleus, similar to how planets orbit the sun. Each shell has a specific energy level and capacity for electrons, with the first shell holding up to 2 electrons, the second up to 8, and so on. The arrangement of electrons is depicted through electron structure diagrams or electron configurations, which vary based on the element's atomic number. The paragraph also introduces the concept of valence electrons, which are the outermost electrons in an atom's shell, and their importance in chemical bonding. Lastly, it mentions the periodic table of elements, which is organized by increasing proton numbers and is fundamental to the study of chemistry.
Mindmap
Keywords
💡Electrons
💡Nucleus
💡Orbitals
💡Electron Shells
💡Atomic Number
💡Electron Configuration
💡Valence Electrons
💡Periodic Table
💡Chemical Bonding
💡Energy Levels
💡Protons
💡Neutrons
Highlights
An atom consists of a nucleus with neutrons and protons, and electrons that move around the nucleus in orbitals.
Orbitals have different shapes based on the number of electrons.
Electrons are arranged in shells similar to planets orbiting the sun.
Each electron shell has a specific energy level and a fixed number of electrons it can hold.
Shells are filled in order of increasing energy levels, starting with the lowest.
The first shell can hold a maximum of 2 electrons, the second shell can hold up to 8.
The third and fourth shells can hold 8 and 2 electrons respectively.
Electron configuration diagrams represent the arrangement of electrons in an atom.
Hydrogen, with an atomic number of 1, has 1 electron filling the first shell.
Carbon, with an atomic number of 6, has 2 electrons in the first shell and 4 in the second.
Sulfur, with an atomic number of 16, has 2 electrons in the first shell, 8 in the second, and 6 in the third.
Calcium, with an atomic number of 20, has a configuration of 2, 8, 8, and 2 electrons across its four shells.
For atomic numbers above 20, the filling pattern of the third shell and beyond changes.
The third shell can fit up to 18 electrons when the atomic number is high.
Valence electrons are the outermost electrons in an atom's shell.
Hydrogen's single electron acts as its valence electron.
Carbon's 4 outer electrons are considered valence electrons.
The periodic table of elements is arranged by increasing proton numbers and is fundamental to chemistry.
Valence electrons play a crucial role in bonding and forming stable compounds.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: