Electron distribution in shells | Structure of an atom | Chemistry | Khan Academy
TLDRThis video script explores the distribution of electrons in the atomic shells of various elements, adhering to specific rules. It begins with the example of a sodium atom, which has 11 electrons distributed in a 2-8-1 pattern across its shells, contrary to a naive assumption of equal distribution. The script introduces the first rule, stating that the maximum number of electrons in any shell is given by the formula 2n^2, where n is the shell number. The second rule, known as the octet rule, limits the outermost shell to a maximum of eight electrons, with exceptions for transition metals. The third rule mandates that electrons fill inner shells completely before occupying outer ones. The script uses additional examples of calcium, carbon, aluminum, and argon to illustrate these principles, encouraging viewers to pause and attempt the electron distribution for aluminum and argon themselves, fostering an interactive learning experience.
Takeaways
- 🔬 Electrons revolve around the nucleus in fixed circular orbits, each with a specific energy level, known as electron orbits or shells.
- 📚 The distribution of electrons across shells follows specific rules, which are explored in the video.
- 💡 The maximum number of electrons in any shell is given by the formula 2n², where n is the orbit number.
- 🚫 The first shell (K shell) can hold a maximum of 2 electrons, and the second shell (L shell) can hold up to 8 electrons.
- 🔶 The outermost shell follows the octet rule, which states it can have a maximum of 8 electrons, with exceptions for transition metals.
- 🌟 Electrons in shells are distributed in a way that inner shells are completely filled before electrons are added to the outer shells.
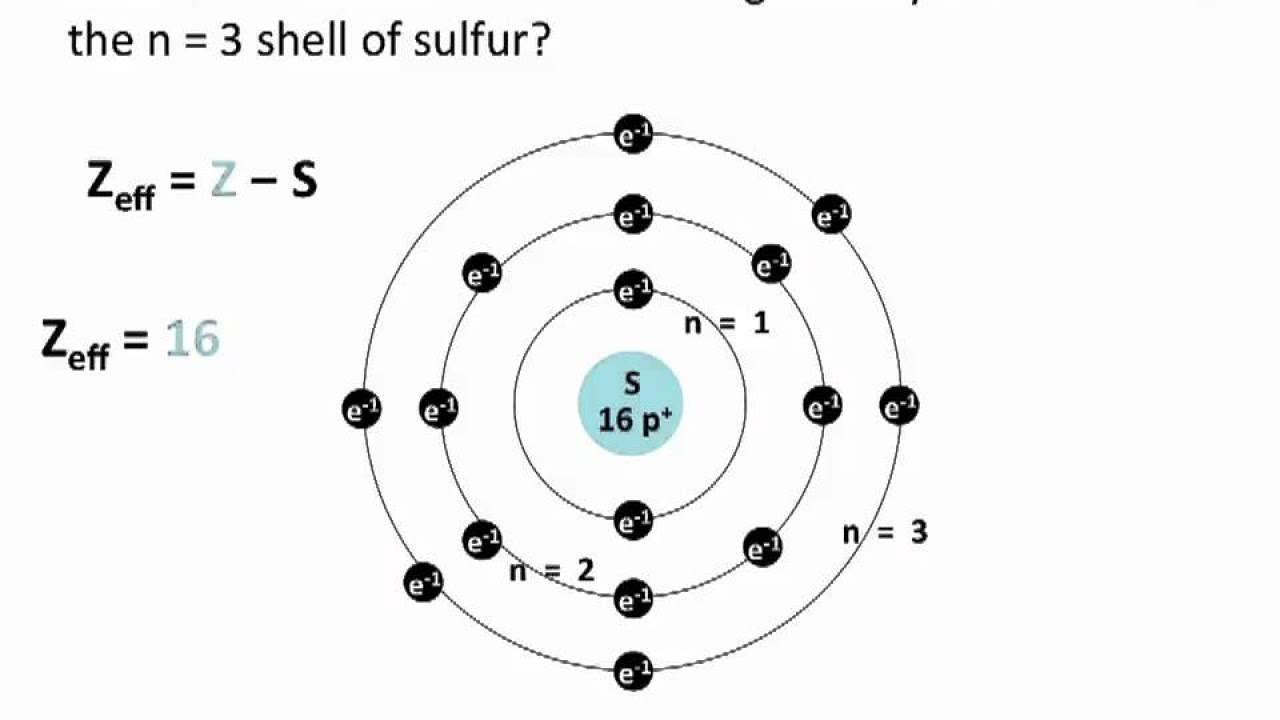
- 🔧 The video uses the example of a sodium atom to illustrate the correct distribution of electrons across shells.
- 🌠 For calcium, the video explains that the third shell (M shell) can hold up to 18 electrons, but follows the octet rule for most elements.
- ⚛ The video clarifies that electrons do not fill a new shell until the previous shells are completely filled, adhering to the octet rule for the outermost shell.
- 🌐 Examples of carbon, aluminum, and argon are provided to demonstrate how electrons are distributed in different atoms.
- ✅ The viewer is encouraged to pause the video and attempt to distribute electrons for aluminum and argon before the video reveals the correct configuration.
Q & A
How do electrons revolve around the nucleus in an atom?
-Electrons revolve around the nucleus in fixed circular orbits, with each orbit having a fixed energy level. These orbits are also known as electron shells.
What is the maximum number of electrons that can be in any shell?
-The maximum number of electrons in any shell is given by the formula 2n^2, where n is the principal quantum number representing the shell or energy level.
How many electrons are in the first shell of a sodium atom?
-In a sodium atom, the first shell (K shell) has a maximum of two electrons.
What is the octet rule in the context of electron distribution?
-The octet rule states that the outermost shell of an atom can have a maximum of eight electrons. This rule is applicable to most elements, except for transition metals.
Why does the third shell of a calcium atom have eight electrons instead of more, given it can hold up to 18?
-The third shell of a calcium atom has eight electrons because it follows the octet rule, which limits the outermost shell to a maximum of eight electrons.
What is the electron configuration of a carbon atom?
-The electron configuration of a carbon atom is 1s² 2s² 2p², with two electrons in the first shell, four in the second shell, and two in the second shell's p subshell.
How many electrons does the first shell of an aluminum atom hold?
-The first shell of an aluminum atom can hold a maximum of two electrons.
What is the total number of electrons in an argon atom?
-An argon atom has a total of 18 electrons, which are distributed across its three shells according to the 2n^2 rule and the octet rule.
Why can't an electron configuration have one electron in the first shell and seven in the second?
-An electron configuration cannot have one electron in the first shell and seven in the second because the inner shells must be completely filled before electrons can be accommodated in the outer shells. The first shell must be filled with two electrons before electrons can be added to the second shell.
What is the significance of the electron distribution in the shells of an atom?
-The electron distribution in the shells of an atom is significant because it determines the chemical properties of the element, including how it will interact with other elements to form compounds.
Do all elements follow the octet rule strictly?
-Most elements follow the octet rule, but there are exceptions, such as transition metals, which can have more than eight electrons in their outermost shell.
What is the electron distribution in the shells of a sodium atom?
-In a sodium atom, the electron distribution is 1s² 2s² 2p⁶ 3s¹, with two electrons in the first shell, eight in the second, and one in the outermost shell.
Outlines
🌟 Electron Distribution in Atoms: Understanding Shells and Energy Levels
This paragraph introduces the concept of electron orbits and the distribution of electrons in these orbits, known as shells. It explains that each shell has a fixed energy level and a specific number of electrons it can hold. The video aims to explore the rules governing electron distribution across these shells. Using sodium as an example, it demonstrates the actual electron distribution in an atom, which is different from a naive assumption of equal distribution. The paragraph also introduces the rule that the maximum number of electrons in any shell is given by the formula 2^n^2, where n is the shell number. This rule is illustrated with examples for the first three shells (K, L, and M), and it explains why the outermost shell in a sodium atom has only one electron.
🔬 Electron Configuration Rules: Octet Rule and Exceptions
This paragraph delves into the rules governing the distribution of electrons in atoms, focusing on the octet rule which states that the outermost shell can hold a maximum of eight electrons. Exceptions to this rule are mentioned, particularly for transition metals like zinc and copper, which can have 18 electrons in their third shell. The paragraph explains that the octet rule is generally followed for most elements. It also addresses why the first shell must be filled with two electrons before electrons are added to subsequent shells, emphasizing that inner shells must be completely filled before electrons are added to outer shells. The concept is further illustrated with examples of carbon, aluminum, and argon, showing how their electrons are distributed according to the established rules.
Mindmap
Keywords
💡Electrons
💡Nucleus
💡Orbits or Shells
💡2n^2 Rule
💡Octet Rule
💡Transition Metals
💡Electron Configuration
💡Inert Gas
💡Chemical Stability
💡Energy Levels
💡Quantum Number
Highlights
Electrons revolve around the nucleus in fixed circular orbits, each with a specific energy level.
Electrons are distributed across shells according to specific rules.
The maximum number of electrons in any shell is given by the formula 2n^2, where n is the orbit number.
The first shell (K shell) can hold a maximum of 2 electrons.
The second shell (L shell) can hold a maximum of 8 electrons.
The third shell (M shell) can hold a maximum of 18 electrons, but follows the octet rule for most elements.
The octet rule states that the outermost shell can have a maximum of 8 electrons.
Transition metals like zinc and copper are exceptions to the octet rule, allowing the third shell to hold up to 18 electrons.
Electrons do not occupy a new shell until the inner shells are completely filled.
The distribution of electrons in a sodium atom follows a specific pattern: 2 in the first shell, 8 in the second, and 1 in the outermost shell.
Calcium's electron distribution is unique, with 2 in the first shell, 8 in the second, 8 in the third, and 2 in the outermost shell.
The distribution of electrons in atoms is guided by the principle that inner shells must be filled before electrons are added to outer shells.
Carbon has 6 electrons distributed as 2 in the first shell and 4 in the second.
Aluminum, with 13 electrons, has a distribution of 2 in the first shell, 8 in the second, and 3 in the third.
Argon, an inert gas with 18 electrons, has a distribution of 2 in the first shell, 8 in the second, and 8 in the third.
The electron distribution in atoms is crucial for understanding chemical properties and reactivity.
Understanding electron shell distribution is fundamental to the study of atomic structure and chemistry.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: