What are States of Matter in Chemistry? - Solid - Liquid - Gas - Plasma - [1-1-2]
TLDRThis lesson delves into the states of matter, focusing on solids, liquids, and gases, and briefly touching on plasma. It explains the properties of each state, such as definite volume and shape, and how they relate to the behavior of molecules. The importance of the polar nature of water molecules is highlighted, emphasizing how it influences properties like boiling points. The lesson also introduces the concept of plasma as a high-energy state of matter, where electrons are separated from atoms, and while it's not central to chemistry, it provides a comprehensive view of matter's various states.
Takeaways
- 📚 The three primary states of matter discussed were solid, liquid, and gas, with a brief mention of the fourth state, plasma.
- 🔬 Elements on the periodic table are the building blocks of matter, with about 118 known elements, though some are man-made and unstable.
- 💡 Atoms and elements are used interchangeably, referring to a unique cluster of protons, neutrons, and electrons.
- 🌀 Molecules are formed when two or more atoms bond together, which can be the same or different elements, and are referred to as compounds when different elements are involved.
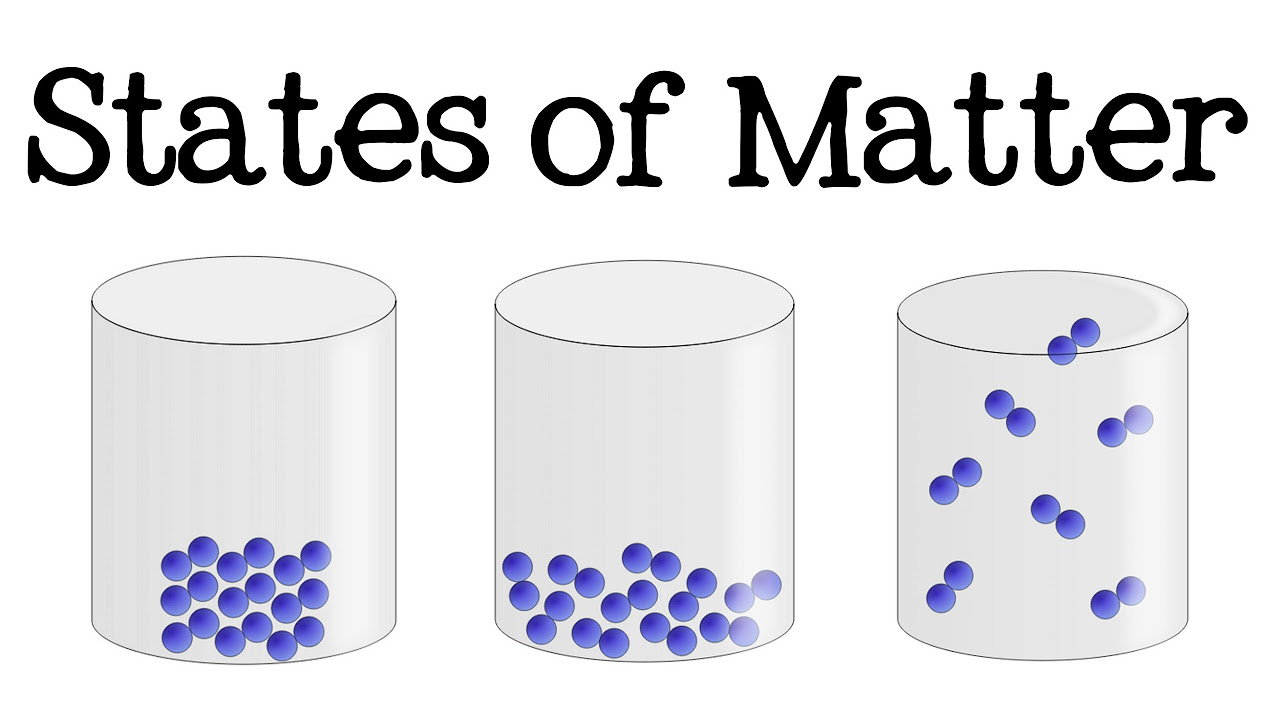
- 🛡️ Solids have a definite volume and shape, are incompressible, and do not conform to the shape of their container.
- 💧 Liquids have a definite volume, can flow, and partially conform to the shape of their container, but are not compressible like solids.
- 🌬️ Gases have no definite volume, are compressible, and expand to fill their container, with the volume being defined by the container's size.
- 🔥 Plasma is a state of matter where atoms are ionized, with electrons detached from their atoms, and is found in stars like the sun.
- 🌊 Water (H2O) is a polar molecule with a slight negative charge on the oxygen end and a slight positive charge on the hydrogen ends, influencing its physical properties.
- 🔄 The electric force, particularly between electrons and protons, is a fundamental aspect of chemistry, being significantly stronger than gravity and governing molecular interactions.
Q & A
What are the three primary states of matter discussed in the lesson?
-The three primary states of matter discussed in the lesson are solid, liquid, and gas.
What is the fourth state of matter mentioned in the lesson?
-The fourth state of matter mentioned in the lesson is plasma, although it is not discussed in detail in the context of chemistry.
How many elements are there on the periodic table?
-There are about 118 elements on the periodic table, although it is often rounded to 100 for simplicity as some elements are man-made and unstable.
What is the difference between an element and an atom?
-An element and an atom are essentially the same thing. An element is a unique cluster of protons, neutrons, and electrons, which is also referred to as an atom.
What is a molecule?
-A molecule is a group of two or more atoms bonded together. This can include the same element bonding to itself or different elements bonding together to form a chemical compound.
What is the main characteristic of solids in terms of their volume and shape?
-Solids have a definite volume and shape. They maintain their shape when placed in a container and are not compressible under normal conditions.
How do liquids behave differently from solids in terms of their response to being placed in a container?
-Liquids take the shape of the container they are placed in, expanding to fill the container but not necessarily the entire volume. They have a definite volume but are not as rigid as solids.
What property of gases is most significantly different from solids and liquids?
-Gases do not have a definite volume and will expand to fill the entire container they are placed in. They are also compressible, unlike solids and liquids.
Why is water considered a polar molecule?
-Water is considered a polar molecule because the oxygen atom pulls the shared electrons slightly closer to itself, creating a slight negative charge on one side of the molecule and a slight positive charge on the other side.
How does the polarity of water molecules affect their behavior in the liquid state?
-The polarity of water molecules causes them to be attracted to each other, with the negative side of one molecule being attracted to the positive side of another. This leads to the formation of temporary attractions between molecules as they slide past each other, influencing properties such as boiling point.
What happens when a plasma is formed?
-A plasma is formed when atoms have so much energy added to them that the electrons are stripped away, leaving behind a charged soup of electrons and positively charged ions. This state of matter is found in stars, including our sun.
Outlines
📚 Introduction to States of Matter
This paragraph introduces the topic of states of matter, specifically solid, liquid, and gas. It briefly mentions the fourth state of matter, plasma, and sets the stage for a deeper understanding of these states. The paragraph emphasizes the importance of understanding what constitutes matter in its various states and reviews the concept of elements and molecules from a previous lesson. It explains that elements are the building blocks of matter and are represented by atoms, which combine to form molecules. The distinction between elements and compounds is clarified, with examples like water (H2O) and methane (CH4) illustrating how different elements can bond to create molecules and compounds.
🌡️ Properties of Solids and Liquids
This paragraph delves into the properties of solids and liquids. Solids are characterized by a definite volume and shape, resistance to compression, and the inability to flow. The paragraph uses the example of a sugar cube to illustrate these points. Liquids, on the other hand, are described as having a definite volume but taking the shape of their container to some extent. They can flow and are not rigidly locked in place due to the close, yet slightly movable, arrangement of their atoms. The paragraph also touches on the incompressibility of liquids, using water as an example to explain that while it might be squeezed a little, it is generally not very compressible.
💨 Understanding Gases
This paragraph focuses on the properties of gases, contrasting them with solids and liquids. Gases are described as having no definite volume and being highly compressible, expanding to fill their container. The paragraph explains that the atoms in a gas are much farther apart compared to solids and liquids, moving rapidly and bouncing off each other and the container walls. This behavior is what allows gases to be compressed and expand to fit their containers. The example of helium in a balloon is used to illustrate these properties of gases.
🧪 Practical Examples of States of Matter
This paragraph applies the concepts of states of matter to real-world examples, examining substances like helium in a balloon, mercury in a thermometer, soup in a bowl, and frozen water (ice). It reinforces the understanding of the properties of solids, liquids, and gases through these practical examples. The paragraph also highlights the importance of recognizing the state of matter to better understand and predict the behavior of substances under different conditions.
🌊 The Polar Nature of Water
This paragraph discusses the polarity of water, explaining how the oxygen atom in a water molecule has a slight negative charge and the hydrogen atoms have a slight positive charge. It describes the sharing of electrons between hydrogen and oxygen atoms, resulting in a polar molecule. The paragraph emphasizes the significance of this polarity in governing water's properties, such as its high boiling point and its ability to dissolve other substances. The structure of ice is also briefly introduced, explaining how the electric force between water molecules leads to the formation of a crystal lattice in solid water.
🌟 The Electric Force in Chemistry
The final paragraph of the script wraps up the lesson by reiterating the importance of the electric force in chemistry. It explains that the electric force, millions of times stronger than gravity, is responsible for the bonding and behavior of molecules. The paragraph introduces the concept of plasma, a state of matter where atoms are ionized and consist of charged particles, and briefly touches on its presence in stars like the sun. The lesson concludes with an encouragement to review the material to fully grasp the fundamental concepts of states of matter and the role of electric forces in chemical interactions.
Mindmap
Keywords
💡States of Matter
💡Elements
💡Molecules
💡Chemical Compounds
💡Atomic Structure
💡Polar Molecules
💡Boiling Point
💡Plasma
💡Electric Force
💡Chemical Bonding
Highlights
The lesson introduces the three common states of matter: solid, liquid, and gas, as well as touching upon the fourth state known as plasma.
There are about 100 elements that make up the bulk of nature, with the heavier elements mostly radioactive and short-lived, thus not discussed in detail in chemistry.
An element is the same as an atom, and together they form a unique cluster of protons, neutrons, and electrons.
Molecules are formed when atoms bond together, which can be the same element or different elements, resulting in compounds when different elements are involved.
Solids have a definite volume and are incompressible, maintaining their shape and size regardless of the container they are placed in.
Liquids can flow and take the shape of their container, but only expand sideways and have a definite volume; they are also incompressible due to the close proximity of their molecules.
Gases have no definite volume and are compressible, expanding to fill whatever container they are placed in.
Plasma is a state of matter where atoms are ionized, with electrons detached from the atoms resulting in a charged soup of particles.
Water (H2O) is a polar molecule with a slight negative charge on the oxygen end and a slight positive charge on the hydrogen ends, influencing many of its physical properties.
The electric force between electrons and protons is millions of times stronger than gravity, and it governs almost all of chemistry at a basic level.
The structure of water, with its bent shape and polar nature, is fundamental to understanding many real-life chemistry phenomena.
When water cools down, the molecules slow down and form a crystal lattice structure in ice due to the strong electric force between them.
The boiling point of a liquid is influenced by the polarity of its molecules, with more polar substances requiring more heat to transition into the gas phase.
The concept of intermolecular attraction and repulsion is crucial for understanding the behavior of substances in different states of matter.
The lesson emphasizes the importance of understanding the basics of chemistry, such as the states of matter and the structure of water, to build a strong foundation for further study.
The polar nature of water molecules leads to the formation of hydrogen bonds, which are essential in many chemical processes and properties of water.
The lesson concludes by reinforcing the significance of the electric force in chemistry and encourages repeated viewing to fully grasp these fundamental concepts.
Transcripts
Browse More Related Video

States of Matter - Solids, Liquids, Gases & Plasma - Chemistry

States of matter follow-up | States of matter and intermolecular forces | Chemistry | Khan Academy
3 States of Matter for Kids (Solid, Liquid, Gas): Science for Children - FreeSchool

Lecture 1 Introduction to Chemistry

States of Matter : Solid Liquid Gas

9.1 Pressure and Kinetic Molecular Theory of Gases | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: