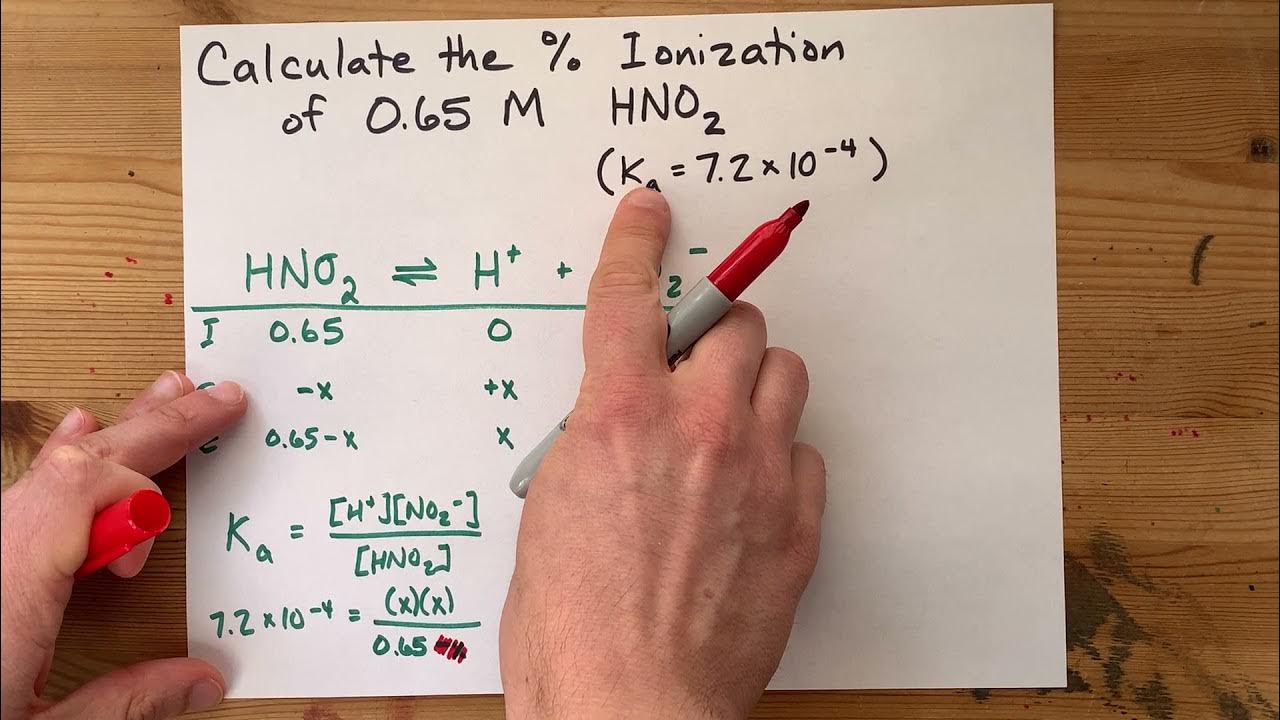
Practice Problem: Calculations Involving pH and Ka
TLDRThe video script presents a step-by-step guide to calculating the Ka value of a weak acid, HNO2, using a pH value and the concept of an ICE table (Initial, Change, Equilibrium). It explains how to set up the equilibrium reaction, account for the stoichiometry, and use the given pH to find the hydronium concentration. The methodical approach leads to the calculation of the Ka value, demonstrating a clear understanding of acid-base equilibrium and chemical calculations.
Takeaways
- 🧪 The script involves calculating the Ka of a weak acid, HNO2, using a pH value and initial molar concentration.
- 📉 Given data includes a 0.0516 M solution of HNO2 with a pH of 2.34.
- 🔄 An acid-base equilibrium reaction is established involving nitrous acid, water, hydronium ions, and nitrite ions.
- 📝 The Ka expression is written in the form of the concentrations of products over reactants, excluding water due to its pure liquid state.
- 🌟 The ICE (Initial, Change, Equilibrium) table is used to organize and calculate the changes in concentration of species involved in the reaction.
- 🎯 Initial concentrations are noted, with 0.0516 M for HNO2 and no hydronium or nitrite ions present.
- 🔢 The change in concentration is represented as -X for HNO2 and +X for both hydronium and nitrite ions, based on the stoichiometry of the reaction.
- 📌 The equilibrium concentrations are calculated by adding the initial and change values for each species.
- 📈 The pH value is related to the hydronium concentration at equilibrium, with a pH of 2.34 corresponding to a hydronium concentration of 4.6 x 10^-3 M.
- 🧠 The value of X is determined to be 4.6 x 10^-3 M, which is used to find the concentrations of hydronium and nitrite ions at equilibrium.
- 🧮 The Ka value is calculated using the equilibrium concentrations, resulting in a Ka of 4.5 x 10^-4 for the acid HNO2.
Q & A
What is the chemical formula for nitrous acid?
-The chemical formula for nitrous acid is HNO2.
What is the concentration of the given nitrous acid solution?
-The concentration of the given nitrous acid solution is 0.516 molar.
What does pH stand for and how is it related to hydronium concentration?
-pH stands for 'potential of hydrogen' and it is a measure of the hydronium ion concentration in a solution. The lower the pH value, the higher the concentration of hydronium ions, indicating a more acidic solution.
What is the pH of the nitrous acid solution mentioned in the script?
-The pH of the nitrous acid solution mentioned in the script is 2.34.
How can you calculate the Ka of a weak acid like nitrous acid?
-You can calculate the Ka of a weak acid like nitrous acid by setting up an acid-base equilibrium calculation, using an ICE (Initial, Change, Equilibrium) table to account for the partial dissociation of the acid, and then solving for Ka using the equilibrium concentrations.
What is the role of water in the dissociation of nitrous acid?
-Water acts as a solvent and a reactant in the dissociation of nitrous acid, accepting a proton (H+) to form hydronium ions (H3O+) and nitrite ions (NO2-).
What is the stoichiometric ratio of reactants to products in the dissociation of nitrous acid?
-The stoichiometric ratio of reactants to products in the dissociation of nitrous acid is 1:1:1, meaning one molecule of HNO2 reacts to form one molecule of H3O+ and one molecule of NO2-.
How do you find the hydronium concentration at equilibrium?
-You find the hydronium concentration at equilibrium by converting the given pH value to its corresponding hydronium concentration using the formula [H3O+] = 10^(-pH). In this case, [H3O+] = 10^(-2.34) M.
What is the calculated Ka value for the nitrous acid solution in the script?
-The calculated Ka value for the nitrous acid solution in the script is 4.5 x 10^(-4).
How does the ICE table help in solving for the Ka of a weak acid?
-The ICE table helps in solving for the Ka of a weak acid by visually representing the initial concentrations, changes in concentration due to the reaction, and the equilibrium concentrations of all species involved. This allows for a systematic approach to solving the equilibrium problem and finding the Ka value.
What is the relationship between the initial concentration of HNO2 and the equilibrium concentrations of H3O+ and NO2-?
-The initial concentration of HNO2 is partially converted into H3O+ and NO2- at equilibrium. The equilibrium concentrations of H3O+ and NO2- are found to be equal to the change in concentration of HNO2, which is 0.0046 M, as calculated from the ICE table.
Outlines
📚 Calculation of Ka for a Weak Acid
This paragraph introduces the task of calculating the Ka value for a weak acid, specifically HNO2, in a solution with a given molarity and pH. The process involves understanding the acid-base equilibrium and setting up an ICE (Initial, Change, Equilibrium) table to account for the dissociation of the weak acid in solution. The paragraph emphasizes the importance of not including water in the equilibrium expression and dealing with coefficients of one for simplicity. The initial concentration of nitrous acid is given, and the goal is to find the Ka value by considering the changes in concentration due to ionization and the final equilibrium state. The pH value is used to determine the hydronium ion concentration at equilibrium, which is then utilized in the ICE table to find the Ka expression.
🧪 Calculation of Ka Using Equilibrium Concentrations
This paragraph focuses on the calculation of the Ka value using the equilibrium concentrations derived from the ICE table. The Ka expression is simplified due to the 1:1:1 stoichiometric coefficients, and the changes in concentration are determined based on the stoichiometry of the balanced equation. The hydronium and nitrite ion concentrations at equilibrium are calculated using the given pH value, and these values are used to fill in the ICE table. The final step is to calculate the Ka value by multiplying the concentrations of the products (hydronium and nitrite ion) and dividing by the concentration of the reactant (nitrous acid). The resulting Ka value is 4.5 x 10^-4, which is a measure of the acid's strength and its tendency to dissociate in solution.
Mindmap
Keywords
💡pH
💡pKa
💡HNO2
💡Ka
💡ICE Table
💡Equilibrium
💡Dissociation
💡Concentration
💡Stoichiometric coefficients
💡Hydronium ion
💡Acid-base equilibrium
Highlights
Calculations involving pH and pKa are discussed, providing insights into acid-base equilibrium.
A weak acid, HNO2, is used in the calculations, emphasizing the behavior of such substances in solution.
The initial setup involves a 0.0516 M solution of HNO2 with a pH of 2.34, highlighting the importance of initial conditions in equilibrium calculations.
The Ka expression is derived, showing the relationship between the concentrations of products and reactants in the equilibrium.
The ICE (Initial, Change, Equilibrium) table method is introduced as a tool for organizing and solving equilibrium problems.
Water is noted as a pure liquid and thus not included in the equilibrium expression, emphasizing the details to consider in chemical calculations.
The stoichiometric coefficients are explained, noting their impact on the changes in concentration during the reaction.
The pH value is related to the hydronium ion concentration, providing a key connection between measured pH and chemical equilibrium.
The hydronium concentration at equilibrium is calculated using the given pH value, demonstrating the practical application of pH in determining ion concentrations.
The equilibrium concentrations of HNO2, hydronium, and nitrite ions are determined using the ICE table and initial conditions.
The Ka value is calculated using the equilibrium concentrations, showcasing the process of finding the acid dissociation constant.
The final Ka value is determined to be 4.5 x 10^-4, illustrating the outcome of the equilibrium calculation.
The tutorial style of the explanation aids in understanding complex chemical concepts, making the subject matter accessible.
The step-by-step approach to solving the problem is emphasized, highlighting the methodical nature of chemical equilibrium calculations.
The importance of understanding the behavior of weak acids in solution is underscored, providing a foundation for further study in acid-base chemistry.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: