AP Chem - Unit 7 Review - Equilibrium in 10 Minutes - 2023
TLDRJeremy Krug's review of AP Chemistry Unit 7 focuses on the concept of equilibrium in reversible processes, highlighting how the rates of forward and reverse reactions determine the balance. The video explains equilibrium constants (Kc and Kp), their temperature dependence, and how to calculate them using initial and equilibrium concentrations. It also discusses the impact of changing conditions on equilibrium, such as concentration, pressure, and temperature, and introduces applications like solubility and the common-ion effect. The review concludes with an overview of entropy and enthalpy changes during dissolution, providing a comprehensive understanding of chemical equilibrium.
Takeaways
- 🌟 Equilibrium refers to reversible processes where the rate of the forward reaction equals the rate of the reverse reaction, resulting in no net change in concentration.
- 📈 The equilibrium constant (Kc or Kp) is expressed as the ratio of concentrations or partial pressures of products over reactants, raised to the power of their stoichiometric coefficients.
- 🔄 Equilibrium processes are dynamic, with forward and reverse reactions occurring simultaneously, not static states.
- 🌡️ The value of the equilibrium constant is temperature-dependent, and changing the temperature can shift the equilibrium position.
- 🔢 The magnitude of the equilibrium constant indicates the extent of the reaction; a large constant suggests a preference for product formation, while a small one indicates a preference for reactant formation.
- 💠 The reaction quotient (Q) is similar to the equilibrium constant but includes values that are not necessarily at equilibrium and can be used to predict the direction in which a reaction will proceed.
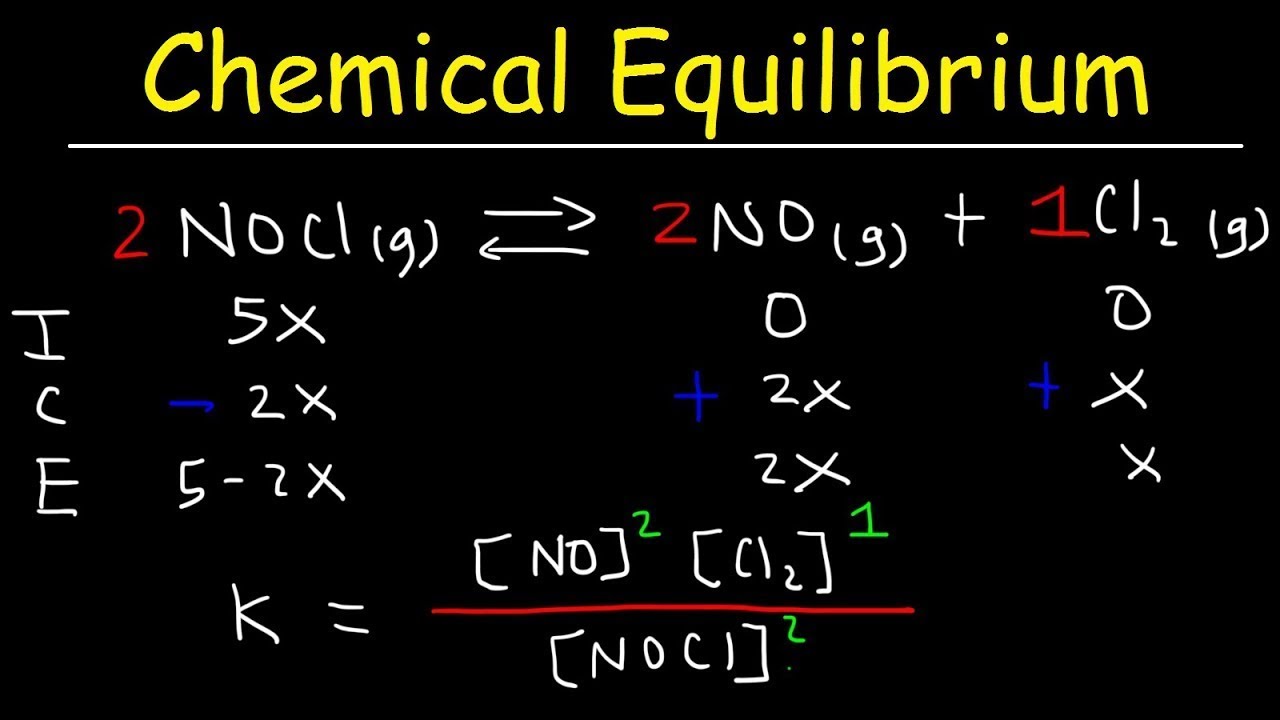
- 📊 An ICE (Initial, Change, Equilibrium) table is a useful tool for keeping track of concentrations and solving for unknown equilibrium concentrations.
- 🔄 If a system at equilibrium is disturbed (e.g., by adding or removing substances, changing volume, or temperature), the system will adjust to re-establish equilibrium.
- 🌊 The common-ion effect demonstrates how the presence of a common ion can affect the solubility of a compound, reducing its ability to dissolve.
- 📉 The solubility product constant (Ksp) is used to express the equilibrium of a compound's dissociation in solution and can be used to calculate the molar solubility of ionic compounds.
- 🔥 Entropy and enthalpy changes during dissolution are important factors; substances typically dissolve with an increase in entropy (disorder), and the process can be either exothermic (release heat) or endothermic (absorb heat).
Q & A
What is the definition of equilibrium in the context of chemistry?
-Equilibrium refers to a state in reversible processes where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in no net change in the concentration of reactants and products over time.
How is reversibility represented in chemical equations?
-Reversibility in chemical equations is represented by a double-headed arrow, indicating that the reaction can proceed in both the forward and reverse directions.
What determines the direction of equilibrium?
-The direction of equilibrium is determined by the relative rates of the forward and reverse reactions. If the forward reaction is faster, more products are formed, and if the reverse reaction is faster, more reactants are present.
How is the equilibrium constant (Kc or Kp) expressed for a reaction?
-The equilibrium constant expression is written as the concentration of the products divided by the concentration of the reactants, each raised to the power of their respective stoichiometric coefficients for Kc, and the partial pressure of the products over the reactants for Kp.
What is the significance of the magnitude of the equilibrium constant?
-The magnitude of the equilibrium constant indicates the extent to which a reversible reaction takes place. A large equilibrium constant suggests that the reaction favors the formation of products, while a small constant indicates that the reaction favors the reactants.
How does temperature affect the equilibrium constant?
-Temperature is the only factor that can change the value of the equilibrium constant. If the temperature changes, the equilibrium constant changes as well, which can shift the equilibrium either towards the products or the reactants.
What is the ICE box used for in equilibrium calculations?
-The ICE (Initial, Change, Equilibrium) box is a tool used to organize data and keep track of the initial concentrations, changes in concentration, and equilibrium concentrations of all species involved in a reaction. It helps in solving for the unknown equilibrium concentrations.
What happens to a system at equilibrium when it is disturbed?
-If a system at equilibrium is disturbed, for example by adding or removing a substance or changing the volume, the system will readjust itself to reach a new equilibrium state. This is known as Le Chatelier's principle.
How does the solubility of ionic compounds change when a common ion is present?
-The presence of a common ion from another compound in the solution reduces the solubility of the ionic compound. This is known as the common-ion effect. For example, adding sodium bromide to a solution containing lead(II) bromide will decrease the solubility of lead(II) bromide due to the increased concentration of bromide ions.
What is the relationship between entropy change (ΔS) and the dissolution of a substance?
-When a substance dissolves, it typically results in an increase in entropy (ΔS) because the orderly solid structure is transformed into a more disordered system of ions or molecules in solution. An increase in entropy means that the total number of possible energy states of the material has increased.
How do enthalpy changes (ΔH) affect the solubility of salts?
-The solubility of salts can be affected by enthalpy changes associated with the dissolution process. If the dissolution is exothermic (releases heat), then adding heat or increasing temperature will generally decrease the solubility of the salt. Conversely, if the dissolution is endothermic (absorbs heat), then increasing temperature will increase its solubility.
Outlines
🌟 Introduction to Equilibrium in AP Chemistry
This paragraph introduces the concept of equilibrium in the context of AP Chemistry, focusing on reversible processes. Jeremy Krug explains that equilibrium is characterized by the forward and reverse reaction rates being equal, resulting in no net change in concentration. He discusses how the direction of equilibrium is influenced by the relative rates of these reactions and introduces the equilibrium constant expressions, Kc and Kp, which are calculated using the concentrations or partial pressures of the reactants and products. The paragraph emphasizes the temperature dependence of equilibrium constants and their implications on the extent of a reversible reaction. Additionally, it covers how equilibrium is affected by manipulating reaction conditions, such as concentration changes, and introduces the ICE box method for calculating equilibrium concentrations.
📊 Understanding Equilibrium through Particle Diagrams and Disturbance
In this paragraph, Jeremy Krug uses a particle diagram to illustrate the concept of equilibrium, showing how the relative numbers of reactants and products can indicate the magnitude of the equilibrium constant. He explains how a system at equilibrium will adjust if disturbed, such as by adding or removing substances, changing volume, or temperature. The discussion then moves to how these changes affect the direction in which the reaction proceeds to re-establish equilibrium, with a focus on the reaction quotient (Q) and its comparison to the equilibrium constant (K). The paragraph also touches on the solubility of ionic compounds, the concept of the solubility product constant (Ksp), and the impact of common ions on solubility, known as the common-ion effect. Additionally, it briefly discusses the solubility of compounds with weak base ions in relation to pH levels.
🔥 Entropy and Enthalpy Changes in Dissolving Processes
The final paragraph delves into the concepts of entropy and enthalpy changes during the dissolution of substances. Jeremy Krug explains that dissolving typically results in an increase in entropy due to the transition from an ordered solid to a disordered system of ions or molecules. He describes how the sign of delta S (∆S) indicates whether the process is entropy-increasing (positive) or not. Similarly, the paragraph discusses the enthalpy change (∆H) during dissolution, highlighting that if heat is released (exothermic process), ∆H is negative, and if heat is absorbed (endothermic process), ∆H is positive. The total free energy of dissolution is also mentioned as a complex factor involving both the solute and solvent molecules. The paragraph concludes with a brief mention of the upcoming review of Unit 8, which covers Acids and Bases.
Mindmap
Keywords
💡Equilibrium
💡Reversible reactions
💡Equilibrium constant (Kc and Kp)
💡Reaction quotient (Q)
💡ICE box
💡Common-ion effect
💡Solubility product constant (Ksp)
💡Entropy
💡Enthalpy
💡Free energy
💡Acid-Base Equilibrium
Highlights
Equilibrium is defined as reversible processes where the forward and reverse reactions occur at the same rate, resulting in constant concentrations of reactants and products.
The direction of equilibrium is determined by the relative rates of the forward and reverse reactions, with the faster reaction influencing the outcome.
The equilibrium constant expression (Kc or Kp) is written as the ratio of the concentrations or partial pressures of the products to the reactants, each raised to the power of their stoichiometric coefficients.
Solids and pure liquids are omitted from equilibrium calculations as their concentrations do not change.
The reaction quotient (Q) is similar to the equilibrium constant but includes values that are not necessarily at equilibrium.
Equilibrium constants are temperature dependent, and changing the temperature is the only way to change the value of the equilibrium constant.
A large equilibrium constant indicates that the reaction favors the formation of products, while a small constant suggests that reactants are favored.
The equilibrium constant can be used to calculate equilibrium concentrations, and an ICE (Initial, Change, Equilibrium) table is recommended for organizing data.
In a particle diagram, the relative numbers of reactants and products can be visually represented to estimate the equilibrium constant.
If a system at equilibrium is disturbed, it will adjust to re-establish equilibrium according to Le Chatelier's principle.
The solubility of ionic compounds is affected by the common-ion effect, where the presence of a common ion reduces the solubility of the compound.
The entropy change (ΔS) during dissolution is typically positive as the substance transitions from an ordered solid to a disordered solution.
The enthalpy change (ΔH) during dissolution can be positive or negative, depending on whether the process is exothermic or endothermic.
The free energy of dissolution is a complex factor involving the energy of both the solute and solvent molecules.
The equilibrium concept is crucial in understanding the solubility of salts and their behavior in the presence of common ions or changes in pH.
The ICE table method is a systematic approach to solving equilibrium problems by tracking initial, change, and equilibrium concentrations.
The equilibrium constant can be manipulated to calculate the concentrations in a reaction that has been reversed or had its coefficients altered.
Transcripts
Browse More Related Video
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc

AP Chemistry Unit 7 Review: Equilibrium!

AP Chem Unit 6 Review - Thermodynamics in 10 Minutes!

AP Chemistry - Unit 7 Exam Review

2022 Live Review 4 | AP Chemistry | Equilibrium Multiple-Choice and Free-Response Questions

Chemistry_Calculating the Equilibrium constant
5.0 / 5 (0 votes)
Thanks for rating: