One Hour Of Mind-Blowing Mysteries Of The Atom | Full Documentary
TLDRThe video script delves into the intricate nature of atoms, exploring their formation, structure, and behavior. It discusses the development of quantum mechanics, the role of electrons, protons, and neutrons, and the forces that govern atomic interactions. The script also addresses fascinating questions about the universe, such as the possibility of the universe being an atom and the journey of atoms after death, highlighting the continuous cycle of matter and the potential near-eternity of certain atoms.
Takeaways
- 🔮 Electrons maintain their orbit around the nucleus through quantized orbits, preventing them from losing energy and collapsing into the nucleus.
- 🌍 The formation of the first atoms post-Big Bang involved processes like nucleosynthesis, leading to the creation of basic elements like hydrogen and helium.
- 💧 Atoms do not truly touch each other; their interactions are governed by electromagnetic forces, making 'touch' a concept more about force interaction than physical contact.
- 🗻 Two atoms of the same element can exhibit differences due to electron states, isotopes, and nuclear excitation, challenging the notion of atomic identity.
- 🌈 The concept of color at the atomic level is a result of light interaction, with atoms themselves not possessing color in a traditional sense.
- 💮 The strong nuclear force keeps protons within an atom's nucleus from repelling each other, overcoming the electromagnetic force.
- 💠 The proton's size was more accurately measured through experiments with muonic hydrogen, revising previous estimates.
- 🚧 Solidity arises from quantum mechanics and electromagnetic forces, explaining how atoms composed mostly of empty space form solid objects.
- ⚒️ Atoms form molecules to reach a lower energy state, driven by quantum mechanics and the desire to achieve a full valence shell.
- ⭐ A neutron star is not a giant atom but exhibits characteristics reminiscent of atomic nuclei, governed by gravity and extreme densities.
- 🌏 The universe's scale and structure inspire theories likening it to an atom in a larger dimension, highlighting the infinity of scale levels.
- ⚰️ Atoms from deceased organisms rejoin the ecosystem, contributing to the cycle of matter and life, showcasing the conservation of matter.
Q & A
How do electrons maintain their motion around the nucleus without slowing down?
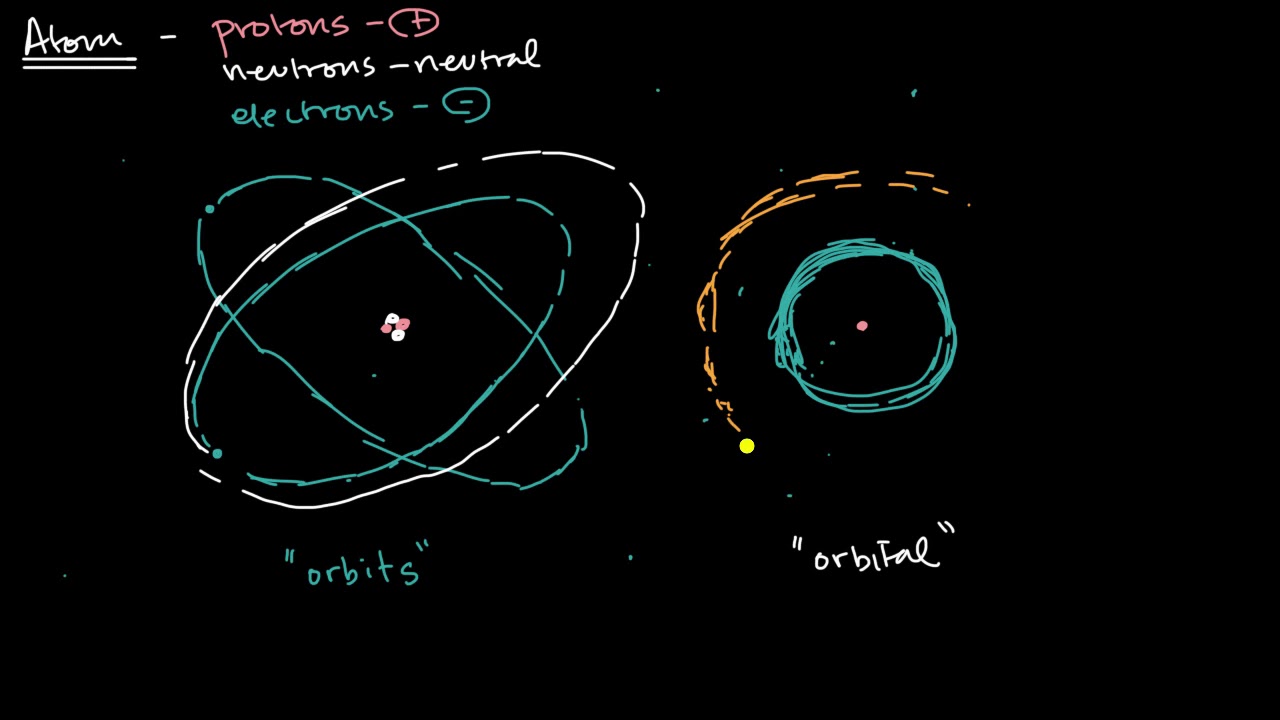
-Electrons maintain their motion around the nucleus due to the principles of quantum mechanics. According to quantum mechanics, electrons exist in specific orbits with defined energy levels and do not emit radiation while in these orbits. This is a result of the wave-like nature of electrons, which form standing waves around the nucleus, similar to how notes are produced on a musical instrument. The stability of these orbits prevents electrons from spiraling inwards and emitting radiation, thus sustaining their motion.
What is the significance of the Big Bang Theory in understanding the formation of atoms?
-The Big Bang Theory is crucial in understanding the formation of atoms as it explains the beginning of the universe. According to this theory, the immense amount of energy condensed after the Big Bang, leading to the formation of atoms. The process involved various epochs such as inflation, quark epoch, and the hadron and lepton epochs, eventually resulting in the formation of protons, neutrons, and electrons, which are the fundamental components of atoms.
How do atoms interact with each other at the microscopic level?
-At the microscopic level, atoms interact primarily through electromagnetic forces. The electrons in the outer shells of atoms repel each other when they come close, preventing the atoms from occupying the same space. However, when atoms are at optimal distances, the electromagnetic force can also facilitate bonding, leading to the formation of molecules. This interaction is not like the solid contact we experience at the macroscopic level but is significant in chemical bonding and reactions.
Why don't protons repel each other out of the nucleus despite being positively charged?
-Protons are kept together in the nucleus by the strong nuclear force, which is much stronger than the electromagnetic force that causes repulsion between the positively charged protons. This strong force acts at very short distances and is responsible for the binding energy within atomic nuclei. It is mediated by particles called gluons, which bind the quarks within protons and neutrons together, overcoming the repulsive electromagnetic force.
How was the size of a proton determined to be smaller than previously thought?
-The size of a proton was determined to be smaller than previously thought through a series of experiments. Initially, two methods were used to measure the proton's radius: one involved observing how electrons interact with the nucleus, and the other measured the energy required to excite an electron from one state to another. However, a discrepancy arose when muons were used instead of electrons, leading to a smaller measured size for the proton. Further experiments using improved precision techniques confirmed the smaller size, resolving the so-called proton radius puzzle.
How can things be solid if atoms are mostly empty space?
-Despite atoms being mostly empty space, the presence of electrons in specific quantized orbits around the nucleus creates a cloud of negative charge that prevents other atoms from passing through. When atoms come close to each other, their electron clouds repel, creating a force that resists further compression. This repulsion is what gives materials their solid properties, as it takes a significant amount of energy to overcome and bring the nuclei of atoms into close contact.
Why do atoms form molecules?
-Atoms form molecules to achieve a state of lower potential energy. When atoms share or transfer electrons, they can attain a full valence shell, which is a state of lower energy and increased stability. This tendency to minimize energy leads to the formation of chemical bonds, such as covalent and ionic bonds, resulting in the formation of molecules and the diverse range of substances we observe.
Is a neutron star similar to one giant atom?
-A neutron star is often described as a giant atomic nucleus due to its composition primarily of neutrons and its extremely dense nature. However, the forces that hold a neutron star together are gravity, which is a result of its immense mass and density, rather than the strong nuclear force that binds protons and neutrons in the nucleus of an atom. While there are similarities in composition, the fundamental forces and scales involved are different.
What if the universe is an atom in a larger reality?
-The idea that the universe could be an atom in a larger reality is a speculative concept that falls under the category of metaphysical or cosmological thought experiments. While there is no scientific evidence to support this notion, it serves as an interesting philosophical question that challenges our understanding of the cosmos and our place within it. It suggests the possibility of a multiverse or higher-dimensional reality where our universe might be just one of many components.
What happens to the atoms of a human body after death?
-After death, the atoms of a human body are recycled back into the ecosystem. Water evaporates or seeps into the ground, eventually becoming part of the water cycle. Soft tissues are metabolized by bacteria, releasing gases like carbon dioxide, nitrogen oxides, and ammonia into the atmosphere. Other atoms are returned to the soil as nutrients, taken up by plants, and eventually consumed by animals and humans. Even trace amounts of radioactive elements decay over time, with some atoms potentially escaping into space and traveling throughout the cosmos.
Do atoms last forever?
-Atoms do not last forever due to processes like radioactive decay, where unstable atoms emit subatomic particles to achieve stability. While some atoms have extremely long half-lives and are effectively stable on a universal timescale, such as bismuth-209, there is ongoing research into the possibility of proton decay, which would suggest that even protons are not truly eternal. However, the timescales involved are so vast that for practical purposes, many atoms can be considered to have near-infinite lifespans.
Outlines
🔬 Exploring Atomic Mysteries
This paragraph introduces a series of thought-provoking questions about the nature of atoms, such as the source of electrons' energy, the formation of the first atom, the uniqueness of atoms of the same element, the possibility of atoms having color, the reasons protons don’t repel out of the nucleus, and the vastness of atoms' empty space. It sets the stage for a deep dive into the fundamental aspects of atomic structure and behavior, challenging common perceptions and encouraging curiosity about the microscopic world that forms the basis of our universe.
⚛️ The Quantum Leap in Understanding Electrons
The second paragraph delves into the quantum mechanics behind electron behavior around an atom's nucleus, highlighting the early 20th-century scientific journey to understanding atomic structure. It discusses the limitations of the solar system model in explaining electron motion, introduces Niels Bohr’s revolutionary theory of quantized electron orbits, and explains how quantum mechanics and Planck’s work on radiation quantization led to a more accurate model of atomic structure. This segment underscores the importance of quantized energy levels in stabilizing atoms and facilitating the complex dance of electrons around the nucleus.
🌌 From the Big Bang to the First Atom
Paragraph three discusses the cosmic origins of atoms, tracing back to the Big Bang and detailing the universe’s evolution from pure energy to the formation of fundamental particles and eventually atoms. It covers the Planck Epoch, the era of cosmic inflation, the unification of forces, and the transition from a quark-gluon plasma to stable atomic structures like protons, neutrons, and electrons. The narrative culminates in the nucleosynthesis process, forming the first stable atoms and leading to the development of complex elements and the chemical diversity we observe today.
🌀 Atomic Interactions and the Illusion of Touch
In paragraph four, the focus shifts to the concept of 'touch' at the atomic level, discussing how atoms, devoid of solid surfaces, interact through electromagnetic forces rather than direct contact. The paragraph elucidates the quantum mechanics principles that define these interactions, illustrating the complexities of atomic and molecular behavior. It explains how the forces between electrons and protons prevent atoms from 'touching' in the conventional sense, yet allow for significant interaction that constitutes the physical basis of our reality.
🌟 The Uniqueness of Atomic Identity
This section addresses whether atoms of the same element are identical. It explores the variability in electron states, the influence of isotopes, and the dynamic nature of atomic components, including protons, neutrons, and electrons. The paragraph highlights how these factors contribute to the distinctiveness of each atom, affecting their behavior in chemical reactions and nuclear processes, and underlining the complexity and uniqueness of atomic identity.
🌈 Do Atoms Have Color?
Paragraph six discusses the concept of color at the atomic level, explaining that atoms do not possess color in the conventional sense because they are smaller than the wavelengths of visible light. It delves into how light interaction with atoms and the resultant absorption and emission spectra give rise to perceived colors, thereby clarifying the distinction between the color of bulk materials and the atomic-level phenomena.
💪 The Power of the Strong Nuclear Force
In paragraph seven, the narrative addresses why protons within the nucleus do not repel each other despite their positive charge. It introduces the strong nuclear force, which overcomes electromagnetic repulsion to bind protons and neutrons together in the nucleus. This explanation underscores the quantum chromodynamics and the intricate balance of forces at play within the atomic nucleus.
🔍 The Proton Size Puzzle
Paragraph eight presents the scientific inquiry into the size of protons, highlighting the discrepancy between measurements derived from electron-proton interactions and those involving muons. The text explains the resolution of this puzzle, where recent experiments aligned the proton size to be smaller than previously believed, demonstrating the complexities and evolving nature of subatomic measurements.
🤔 Solidity in an Atomically Empty Space
This section explores the paradox of solidity in materials composed mostly of empty space at the atomic level. It describes how the interactions of electron clouds within atoms prevent physical objects from passing through each other, despite the vast empty spaces within atoms, providing a fundamental explanation for the perceived solidity of matter.
🔗 The Chemistry of Bonding
Paragraph ten explains why atoms form molecules, emphasizing the role of energy and quantum mechanics in the process. It details how atoms achieve lower energy states and stability through bonding, forming molecules that constitute the diverse chemical structures and materials in the universe.
🌟 Neutron Stars: Cosmic Giants
This paragraph examines the nature of neutron stars, comparing them to giant atomic nuclei and explaining the forces and processes involved in their formation. It highlights the extreme conditions, such as immense density and gravitational force, under which these stars exist, offering a glimpse into the extraordinary characteristics of these cosmic entities.
🌌 Contemplating a Universal Atom
Paragraph twelve speculates on the concept of the universe as an atom within a larger dimensional scale, touching on theories like the one-electron universe and the multiverse. It explores the idea of infinite scalability in the cosmos and how our universe could be analogous to an atomic structure in a grander scheme of existence.
♻️ The Eternal Journey of Atoms
In the thirteenth paragraph, the focus is on the lifecycle of atoms after death, illustrating how the conservation of matter leads to the continuous recycling of atoms in nature. It details how the atoms that constitute living beings are redistributed in the ecosystem, contributing to the ongoing cycle of matter and life on Earth.
⏳ Do Atoms Endure Forever?
The final paragraph delves into the longevity of atoms, discussing the concept of radioactive decay and the potential for proton decay as avenues for atomic transformation. It contemplates the near-eternal existence of certain atoms like bismuth-209 and the broader question of whether any part of matter, including protons, can truly last forever.
Mindmap
Keywords
💡Quantum Mechanics
💡Big Bang Theory
💡Electromagnetic Force
💡Isotopes
💡Strong Nuclear Force
💡Half-life
💡Subatomic Particles
💡Proton Decay
💡Covalent Bonds
💡Neutron Stars
Highlights
Electrons sustain motion around the nucleus through quantum mechanics, challenging early 20th-century physics.
Niels Bohr proposed quantized orbits for electrons in 1913, laying the groundwork for quantum mechanics.
Quantum mechanics reveals electrons exhibit both particle and wave behavior, leading to standing wave patterns around the nucleus.
Atoms form because of the balance between electrical attraction and kinetic energy of electrons.
The universe's first atoms formed from the Big Bang's primordial soup, setting the stage for cosmic evolution.
Atoms never actually touch each other due to the electromagnetic force, redefining our understanding of 'touch'.
Two atoms of the same element can be different due to electron states, motion, and nuclear composition.
Atoms do not inherently have color; color perception is a result of light interaction with atom assemblies.
The strong nuclear force keeps protons in the nucleus from repelling each other, crucial for atom stability.
The proton radius puzzle challenged physicists' understanding, resolved to show protons are smaller than previously thought.
Matter's solidity is due to the quantum mechanics of electrons, not the physical filling of space.
Atoms bond to form molecules due to energy minimization, fundamental to chemical diversity.
Neutron stars are akin to giant atomic nuclei, showcasing extreme conditions and forces.
The concept of the universe as an atom in a larger dimension challenges perceptions of reality.
Atoms from deceased organisms rejoin the ecosystem, contributing to life's continuity.
The potential for proton decay and the eventual fate of atoms remain subjects of profound scientific inquiry.
Transcripts
Browse More Related Video

How Did Atoms Form From Nothing?

Basic Atomic Structure: A Look Inside the Atom
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy

What Is An Atom - Part 1 | Properties of Matter | Chemistry | FuseSchool

ALL OF PHYSICS explained in 14 minutes

Introduction to the atom | Chemistry of life | Biology | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: