Atomic Mass: Introduction
TLDRThe video script introduces the concept of atomic mass, emphasizing its importance as an element characteristic and explaining it as a weighted average. Using an analogy with different car models, the script clarifies how isotopes of an element, like Copper 63 and Copper 65, contribute to the atomic mass based on their abundance. The detailed calculation process for weighted averages is outlined, demonstrating how the atomic mass of copper is derived, and noting that real-world calculations involve more precise numbers than those used in the simplified example.
Takeaways
- 📚 Atomic mass is a crucial characteristic for elements, representing the average mass of an element's atoms.
- 🌟 Each element has a unique atomic mass, which is depicted as a number below the element's symbol on the periodic table.
- 🔍 Atomic mass is determined using a weighted average, which accounts for the different isotopes of an element and their relative abundances.
- 🚗 The concept of a weighted average is explained using an analogy of different car models with varying weights and abundances.
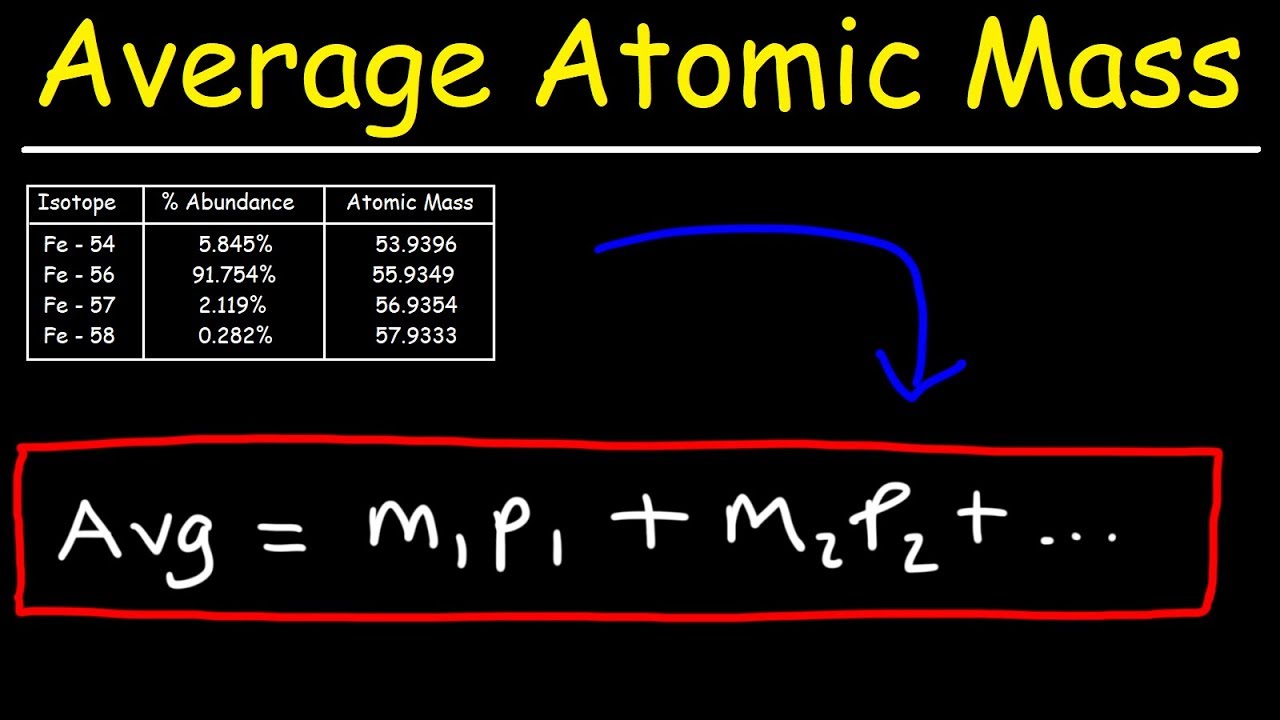
- 📈 To calculate a weighted average, multiply each option (isotope or car model) by its percentage abundance and then sum these values.
- 🏷️ Isotopes of an element have the same number of protons but differ in their number of neutrons, similar to how different car models share a common design.
- 🧪 The atomic mass of copper is used as an example, with two isotopes: Copper 63 and Copper 65, having different masses and abundances.
- 🎯 Copper's atomic mass is approximately 63.62 amu, which is a weighted average of the masses and abundances of its isotopes.
- 🤔 The atomic mass listed on the periodic table is a precise weighted average, taking into account more exact values than used in the example.
- 📊 Understanding weighted averages is essential for grasping how atomic masses are calculated and represented in chemistry.
- 📝 The video script serves as an educational tool to illustrate the concept of weighted averages and their application in calculating atomic masses.
Q & A
What is atomic mass and why is it important?
-Atomic mass is a characteristic of elements that represents the average mass of atoms of an element. It is important because it is used in the periodic table and is essential for understanding chemical reactions and the properties of substances.
How is atomic mass different from the kind of average you might have learned in math?
-Atomic mass is a weighted average, which takes into account the relative abundance of isotopes, rather than a simple average that equally considers all items in a set.
What is an analogy used in the script to explain weighted averages?
-The script uses the analogy of two models of a car called Lemona (GX and GXL) with different features and weights to explain weighted averages.
What are isotopes and how do they relate to the Lemona car models?
-Isotopes are variants of an element that have the same number of protons but different numbers of neutrons. The Lemona car models (GX and GXL) are analogous to isotopes in that they share a common characteristic (the lemon-like shape) but have different features and masses.
How do you calculate the weighted average for the Lemona cars?
-To calculate the weighted average for the Lemona cars, you multiply the weight of each model by its percent abundance (converted to a decimal) and then add the results together.
What is the mass of Copper 63 and Copper 65 isotopes?
-The mass of Copper 63 is approximately 63 amu, and the mass of Copper 65 is approximately 65 amu.
What are the approximate abundances of Copper 63 and Copper 65 isotopes?
-Copper 63 is approximately 69% abundant, and Copper 65 is approximately 31% abundant.
How does the weighted average of Copper isotopes differ from a simple average?
-The weighted average of Copper isotopes (63.62 amu) is closer to the mass of the more abundant isotope (Copper 63), while a simple average would be exactly halfway between the two isotopes' masses (64 amu).
Why does the atomic mass on the periodic table differ slightly from the calculated weighted average?
-The atomic mass on the periodic table is more precise because it uses the exact masses and abundances of isotopes, whereas the calculated weighted average used rounded numbers for simplicity.
What is the significance of understanding weighted averages in chemistry?
-Understanding weighted averages is crucial in chemistry for calculating atomic masses and for comprehending the behavior of elements and compounds, especially when dealing with isotopes and their relative abundances.
How can the concept of weighted averages be applied to real-world scenarios?
-The concept of weighted averages can be applied to various real-world scenarios where the distribution of different items or values is not uniform, such as in finance, statistics, and environmental studies.
Outlines
🔬 Introduction to Atomic Mass and Weighted Averages
This paragraph introduces the concept of atomic mass, emphasizing its importance in the characterization of elements. It explains that atomic mass is an average value, specifically a weighted average, which is different from the regular average taught in math. The paragraph uses an analogy of two car models, the Lemona GX and GXL, to illustrate the concept of weighted averages. It highlights how the atomic number defines an element, similar to how the shape of a Lemona defines the car model, and how isotopes of an element differ in neutron numbers but not in proton numbers.
📊 Understanding Weighted Averages with the Lemona Analogy
The paragraph delves deeper into the concept of weighted averages by continuing the Lemona car analogy. It explains how to calculate the weighted average by taking into account the abundance of each car model. The example demonstrates that the average weight of Lemonas is closer to the weight of the more abundant GX model rather than being exactly in the middle of the two models' weights. The paragraph then transitions to discussing isotopes of elements, using Copper's isotopes as an example, and how their masses and abundances contribute to the atomic mass of the element.
🧪 Calculation of Atomic Mass with Real Isotope Data
This paragraph explains the process of calculating atomic mass using real data for Copper isotopes. It shows how to apply the concept of weighted averages to isotopes by considering both their masses and their abundance in nature. The example calculates the weighted average mass of Copper as 63.62 amu, which is closer to the mass of the more abundant isotope, Copper 63. The paragraph also addresses the discrepancy between the calculated atomic mass and the one found on the periodic table, attributing it to the use of rounded numbers in the example and explaining that using precise values would yield an atomic mass that matches the periodic table.
Mindmap
Keywords
💡Atomic Mass
💡Weighted Average
💡Isotopes
💡Abundance
💡Lemona Cars
💡Atomic Number
💡Periodic Table
💡Copper
💡amu (Atomic Mass Units)
💡Practice Problems
Highlights
Atomic mass is a key characteristic for elements, representing the number written underneath the element's symbol on the periodic table.
Atomic mass is a weighted average, a special kind of average that takes into account the abundance of different isotopes of an element.
The concept of isotopes is introduced, comparing them to different models of a car with the same basic features but varying additional characteristics.
Copper serves as an example, having two isotopes, Copper 63 and Copper 65, with the same number of protons but different numbers of neutrons.
The average weight of two different cars (analogous to isotopes) is calculated using a regular average and then a weighted average to demonstrate the difference.
In the weighted average calculation, the Lemona GX (representing a lighter isotope) is more abundant, thus more significantly impacts the average weight.
The weighted average is used to calculate the atomic mass of an element by considering both the mass and the abundance of its isotopes.
The mass of Copper 63 is approximately 63 amu, and Copper 65 is approximately 65 amu, but actual values are slightly different for calculation purposes.
The abundance of Copper 63 is about 69%, and Copper 65 is about 31%, which is used to calculate the weighted average of copper's atomic mass.
The calculated weighted average atomic mass of copper is 63.62 amu, which differs from the rounded value on the periodic table.
The discrepancy in calculated and rounded atomic mass values is due to the use of cleaner numbers for ease of understanding during the initial calculation.
The actual abundance and mass of Copper 63 and 65 are 69.17% and 62.93 amu, and 30.83% and 65.08 amu respectively.
When precise values are used in the weighted average calculation, the resulting atomic mass matches the value found on the periodic table.
Understanding weighted averages and their application to isotopes is crucial for calculating accurate atomic masses.
The video provides a clear analogy and step-by-step explanation of how to calculate weighted averages and apply them to atomic mass.
The concept of abundance is introduced as a key factor in determining the weighted average of atomic masses.
The video concludes by encouraging viewers to practice calculating weighted averages and atomic masses with other elements.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: