Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (74 of 92) R=? T=? V0=(3/4)E,.(Ex. 4 of 4)
TLDRThis lecture explores the behavior of particles encountering potential steps in a quantum mechanics context. As the step's energy approaches that of the particle, reflection coefficients are calculated to determine the percentage of particles reflected. Intriguingly, even when the potential step's energy is 99% of the particle's energy, only 66.9% of particles are reflected, highlighting the counterintuitive nature of quantum mechanics compared to classical mechanics.
Takeaways
- 📈 When the potential step's energy approaches the particle's energy, the reflection coefficient changes significantly.
- 🔢 At 3/4 the energy of the particle, 11.1% of particles are reflected.
- 🔽 As the potential step's energy increases to 90% of the particle's energy, the reflection coefficient rises to 27%.
- 🔼 Conversely, 73% of particles are transmitted when the potential step is at 90% of the particle's energy.
- 🌟 At 99% energy equivalence, a surprising 66.9% of particles are reflected, with only 33.1% transmitted.
- 💡 Quantum mechanics differs from classical mechanics in how particles interact with potential steps.
- 🚧 Even when the potential step's energy is less than the particle's energy, quantum mechanics predicts reflection.
- 📊 The reflection coefficient is calculated using the formula 1 - (1 - (potential/energy)^2)^0.5.
- 🌐 The results are a direct application of quantum tunneling, a phenomenon not accounted for in classical physics.
- 🤔 The lecture explores the boundary between quantum and classical behavior in terms of particle energy and potential steps.
- 📚 The script serves as an educational resource for understanding the principles of quantum mechanics and their implications.
Q & A
What is the main topic of the lecture?
-The main topic of the lecture is the behavior of particles when encountering a potential step in quantum mechanics, specifically how the reflection coefficient changes as the potential step's energy approaches the particle's energy.
How does the potential step's energy relate to the particle's energy in the first case discussed?
-In the first case, the potential step's energy is 3/4 (75%) of the particle's energy.
What is the formula used to calculate the reflection coefficient?
-The formula used to calculate the reflection coefficient is R = (1 - (V/E)^2) / (1 + (V/E)^2), where V is the potential step's energy and E is the particle's energy.
What percentage of particles are reflected when the potential step is 3/4 the energy of the particle?
-When the potential step is 3/4 the energy of the particle, approximately 11.1% of the particles are reflected.
How does the reflection coefficient change when the potential step's energy is 90% of the particle's energy?
-When the potential step's energy is 90% of the particle's energy, the reflection coefficient is approximately 27%.
What is the classical mechanics expectation for particle behavior when the potential step's energy is less than the particle's energy?
-In classical mechanics, if the potential step's energy is less than the particle's energy, 100% of the particles would continue past the step without reflection.
What happens when the potential step's energy is 99% of the particle's energy in quantum mechanics?
-When the potential step's energy is 99% of the particle's energy, about 66.9% of the particles are reflected, and approximately 33.1% are transmitted.
How does quantum mechanics differ from classical mechanics in the case where the potential step's energy is 99% of the particle's energy?
-In quantum mechanics, with the potential step's energy at 99% of the particle's energy, 33.1% of the particles are still transmitted, whereas in classical mechanics, 100% of the particles would be transmitted with no reflection.
What is the significance of the reflection coefficient in quantum mechanics?
-The reflection coefficient in quantum mechanics is significant as it quantifies the probability of a particle being reflected by a potential step, which is a key concept in understanding wave-particle duality and quantum behavior.
How does the potential step affect the transmission of particles in quantum mechanics?
-In quantum mechanics, the potential step affects the transmission of particles by altering their wavefunction, which in turn affects the probability of reflection and transmission. As the potential step's energy approaches the particle's energy, the reflection coefficient increases, and the transmission decreases.
What is the role of the potential step in quantum mechanics?
-The potential step in quantum mechanics serves as a model to study the wave-like behavior of particles. It helps in understanding how particles interact with energy barriers and how their behavior differs from classical mechanics, particularly in terms of reflection and transmission probabilities.
Outlines
🌟 Quantum Mechanics and Particle Reflection
This paragraph delves into the behavior of particles in the realm of quantum mechanics, particularly focusing on how the energy of a potential step affects the reflection coefficient. The discussion begins with a scenario where the potential step's energy is 3/4 that of the particle, then progresses to 90% and finally 99% of the particle's energy. Through calculations using a simplified equation, it's revealed that as the potential step's energy approaches that of the particle, the percentage of reflected particles decreases. Notably, when the potential step is 99% the energy of the particle, only 66.9% of the particles are reflected, contrasting with classical mechanics where 100% of particles would be transmitted. This highlights the intriguing and counterintuitive nature of quantum mechanics.
Mindmap
Keywords
💡Potential Step
💡Reflection Coefficient
💡Quantum Mechanics
💡Energy
💡Particle
💡Transmission
💡Classical Mechanics
💡Square Root
💡Fraction
💡Wave-Particle Duality
💡Quantum Superposition
Highlights
The lecture explores the impact of increasing the potential step's energy relative to the particle's energy on the reflection coefficient.
The potential step's energy is initially set at 3/4 the energy of the particle to observe the reflection.
When the potential step is 3/4 the energy of the particle, 11.1% of the particles are reflected.
The potential step's energy is then increased to 90% of the particle's energy.
At 90% energy equivalence, only 27% of the particles are reflected, while 73% are transmitted.
In classical mechanics, all particles would pass the step if the potential energy is less than the particle's energy.
The potential step's energy is further increased to 99% of the particle's energy.
At 99% energy equivalence, 66.9% of the particles are reflected, and 33.1% are transmitted.
In contrast to quantum mechanics, in classical physics, all particles would be transmitted at 99% energy equivalence.
The quantum mechanics model shows that even when the potential step's energy is greater than the particle's energy, a significant number of particles are still transmitted.
The reflection coefficient is calculated using the simplified equation 1 - sqrt(1 - (potential energy / particle energy)^2).
The lecture demonstrates the counterintuitive nature of quantum mechanics compared to classical mechanics.
The study provides insights into particle behavior at energy thresholds in quantum states.
The results show that quantum mechanics allows for particle transmission even when facing high energy barriers.
The lecture's findings are a practical application of quantum mechanics principles.
The mathematical approach to calculating reflection coefficients is detailed for different energy ratios.
The lecture's content is a valuable resource for understanding quantum mechanics' unique behaviors.
Transcripts
Browse More Related Video
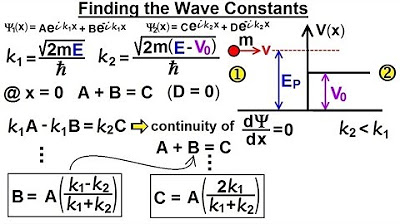
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (65 of 92) Wave Constants B=? C=?
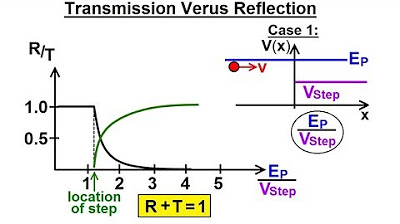
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (62 of 92) Transmission vs Reflection
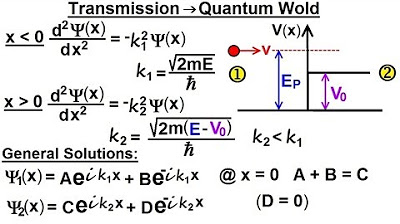
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (64 of 92) Transmission-Reflection (Q.M.)
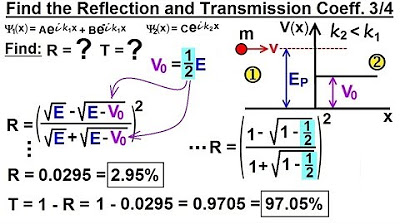
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (73 of 92) R=? T=? V0=(1/2)E (Ex. 3 of 4)
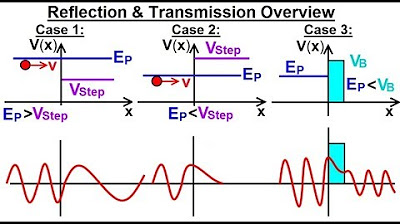
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (61 of 92) Reflection & Transmission
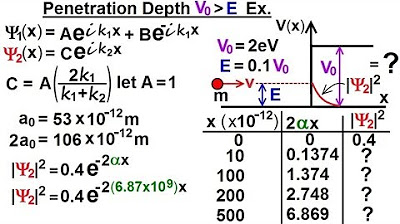
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (76 of 92) Penetration Depth V0, E: Ex.
5.0 / 5 (0 votes)
Thanks for rating: