Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (24 of 92) Prob. of Finding Particle 4
TLDRIn this educational video, the concept of finding the probability of a particle's location is explored using a one-dimensional model. The video demonstrates how, as the interval Delta X becomes infinitesimally small, the probability at a specific point converges to a precise value. By squaring the wave function and multiplying it by a small Delta X, the probability at any given point is determined. This method simplifies the process of calculating probabilities along a particle's path and is applicable to more complex models, offering a clear and efficient approach to understanding quantum behavior.
Takeaways
- 📈 The probability of finding a particle between L/4 and L/4 + ΔX was calculated to be 0.01032 with ΔX趋向于0.
- 🔄 As ΔX becomes smaller, the 3/2 term in the probability expression diminishes, approaching zero in the limit.
- 🎯 The script introduces an alternative method for finding the probability of a particle's location when ΔX is sufficiently small.
- 🌟 The alternative method involves using the probability function (wave function squared) multiplied by a very small ΔX to find the exact probability.
- 📌 For a one-dimensional model, the probability function is derived from the wave function squared, specifically 2/L * sin^2(nπx/L) with n=1 for the lowest energy level.
- 🔢 The example given uses ΔX = 0.01L and finds the probability at location L/4, resulting in a probability P = 0.01.
- 🥢 The script demonstrates that the new method yields the same result as integrating the probability function over specific limits.
- 🌐 This approach can be generalized for more complex models beyond the simple one-dimensional case presented.
- 📊 The method provides a slick and efficient way to determine the probability of finding a particle at any point along its path.
- 🚀 As X approaches 0, the 3/2 term becomes negligible, confirming the consistency of the method with previous results.
- 📋 The script serves as an educational tool for understanding quantum mechanics and the calculation of probabilities in particle physics.
Q & A
What was the probability found in the previous video for a particle being between L/4 and L/4 + ΔX?
-The probability found was 0.01032, with ΔX being a small value.
How does the probability change as ΔX approaches zero?
-As ΔX approaches zero, the probability tends to zero, following the 3/2 term in the expression.
What is the alternative method introduced for finding the probability of finding a particle at a particular location?
-The alternative method involves using the probability function (wave function squared) multiplied by a very small ΔX to find the probability at a specific location.
What is the significance of using the wave function squared in this context?
-The wave function squared represents the probability density, which when multiplied by a small interval ΔX, gives the probability of finding the particle within that interval.
What is the role of the constant 2/L in the probability calculation?
-The constant 2/L normalizes the probability function, ensuring that the total probability over the entire range equals 1 for a normalized wave function.
Why is the value of N set to 1 in this calculation?
-N is set to 1 because the calculation is focused on the innermost energy level, or the lowest energy level, where the quantum number n is equal to 1.
What is the value of ΔX used in the example calculation?
-In the example, ΔX is set to 0.01L, representing a very small interval along the particle's path.
What is the location X chosen for the probability calculation in the example?
-The location X chosen is L/4, which corresponds to the midpoint of the interval between L/4 and L/4 + ΔX.
What is the result of the probability calculation at X = L/4?
-The probability at X = L/4 is 0.005 (or 0.01 times 0.5), which is the chance of finding the particle at that specific location.
How does this method simplify the process of finding probabilities along a path?
-This method simplifies the process by directly using the probability function and a small interval ΔX, eliminating the need to calculate integrals over specific limits.
What happens to the 3/2 term in the probability expression as ΔX becomes infinitesimally small?
-As ΔX becomes infinitesimally small, the 3/2 term disappears, leading to the same result obtained from integrating the probability function over the interval.
Outlines
📊 Quantum Probability and the Electron Line
This paragraph introduces the concept of finding the probability of a particle's location in a quantum context. It discusses the previous video's findings where the probability of finding a particle between L and L + ΔX was calculated as 0.01032. The speaker explains that as ΔX approaches zero, the probability tends to a fixed value of 0.01. The paragraph then transitions to a new method for determining the probability of finding a particle at a specific location by using the probability function and multiplying it by a very small ΔX. The example given uses a one-dimensional model and a sine squared function to illustrate how the probability is calculated for the lowest energy level. The key takeaway is that this method provides the same result as integrating the probability function over a range, offering a simpler approach to finding probabilities at any point along the particle's path.
Mindmap
Keywords
💡probability
💡integral
💡wave function
💡Delta X
💡quantum mechanics
💡energy levels
💡sine squared
💡one-dimensional well
💡quantization
💡limits
💡calculus
Highlights
The probability of finding a particle between L/4 and L is calculated as 0.01032.
As Delta X approaches zero, the probability tends towards a specific value.
An alternative method for finding the probability of a particle's location is introduced when Delta X is sufficiently small.
The probability function is replaced by the wave function squared to find the probability.
For a one-dimensional well, the wave function squared is used to calculate the probability.
The probability is given by (2/L) * sine squared(n PI x/L) * Delta X.
N equals 1 is used for the lowest energy level in the calculations.
The probability at L/4 is calculated to be 0.01.
The method simplifies the process of finding the probability at any point along the path.
The result is consistent with the integral method when Delta X approaches zero.
This approach can be applied to more complex models in the future.
The sine squared function is used to determine the probability at a specific location.
The probability calculation is demonstrated with Delta X set to 0.01 L.
The final probability value is 0.005, illustrating the effectiveness of the method.
This method provides a slick way to find the particle's probability at any point.
The process is explained step by step, making it easy to understand and replicate.
Transcripts
Browse More Related Video

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (10 of 92) What is Normalization? Ex. 1
How Derivatives Arise from Limits – Topic 50 of Machine Learning Foundations
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (18 of 92) Particle in 1-D Box: Gen. Appr.
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (9 of 92) What is the Prob Distribution?

Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (27 of 92) Expectation Value=? 1-D Box n=1
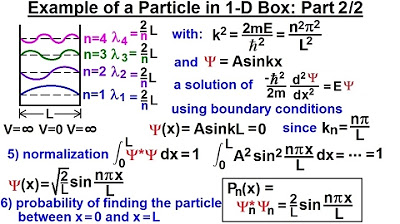
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (20 of 92) Particle in 1-D Box: Example 2/2
5.0 / 5 (0 votes)
Thanks for rating: