Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (8 of 92) Meaning of the Wave Eqn.
TLDRThe video delves into the physical meaning of the wave equation and its role in quantum mechanics. It explains that the wave equation, which is not a real quantity, allows for the calculation of the probability of finding a particle at a specific location. The script clarifies that while the wave equation does not represent a physical wave or a real function, it is a crucial tool in quantum mechanics for understanding particle behavior and probabilities.
Takeaways
- 🌊 The wave equation is a fundamental concept in quantum mechanics, representing the physical behavior of particles under certain constraints.
- 📐 The script begins by discussing the Schrödinger equation, which is a differential equation that operates on the wave function.
- 🔄 The wave equation should satisfy the Schrödinger equation, just like any solution must satisfy its corresponding differential equation.
- 🎲 The wave equation is initially introduced in the context of a particle with mass M in a single dimension, subject to various physical forces and energies.
- 🌀 The wave equation is written as a function of position and time, and when plugged into the Schrödinger equation, it should satisfy it.
- 📈 The script presents a specific form of the wave equation involving a constant, e to the power of (I KX - Omega T), where I is the imaginary unit.
- 🚫 Despite its appearance, the wave equation does not represent a real, measurable quantity or a classic wave function in quantum mechanics.
- 🎱 The wave equation is crucial for calculating the probability of finding a particle in a particular location, rather than representing the particle's position or movement directly.
- 📊 The script clarifies that the wave equation, in its complex form, is a vehicle for calculating probabilities in a one-dimensional case.
- 🔑 The technique for using the wave equation to calculate probabilities will be explained in subsequent videos.
- 🤔 The concept that the wave equation does not represent a real wave or quantity is a challenging aspect of quantum mechanics, but it is essential for understanding particle behavior.
Q & A
What is the primary purpose of the wave equation in quantum mechanics?
-The primary purpose of the wave equation in quantum mechanics is to provide a means to calculate the probability of finding a particle in a particular location, rather than representing a measurable classical wave function.
How is the wave equation related to the Schrödinger equation?
-The wave equation satisfies the Schrödinger equation, which is a fundamental differential equation in quantum mechanics that describes how the quantum state of a physical system changes with time.
What does the wave equation represent in the context of a single particle in a one-dimensional environment?
-In the context of a single particle in a one-dimensional environment, the wave equation represents the quantum state of the particle, which is a complex-valued function of position and time.
Why does the wave equation contain the imaginary unit 'i'?
-The imaginary unit 'i' is present in the wave equation because it allows for the representation of complex numbers, which are necessary for describing quantum states that cannot be represented by real numbers alone.
What happens to the wave equation when there are no forces acting on the particle and the potential energy is constant?
-When there are no forces acting on the particle and the potential energy is constant, the Schrödinger equation reduces to a simpler form, and the wave equation can be used to calculate the probability distribution of the particle's location.
How does the wave equation differ from classical wave equations?
-The wave equation in quantum mechanics is not a real quantity and does not represent a physical wave like classical wave equations do. Instead, it provides a way to calculate probabilities in quantum systems.
What is the significance of the probability aspect in the wave equation?
-The probability aspect is significant because it allows us to predict the likelihood of finding a particle at a specific location, which is a core concept in understanding quantum behavior and is different from classical physics where the exact position and momentum of a particle can be precisely known.
What does the wave equation look like for a particle with no net force acting on it?
-For a particle with no net force acting on it, the wave equation takes the form of a constant times e to the power of (I KX - Omega T), where I is the imaginary unit, K is the wave number, X is the position, Omega is the angular frequency, and T is time.
How can the wave equation be expressed in terms of cosine and sine functions?
-The wave equation can be expressed in terms of cosine and sine functions by using Euler's formula, which allows the transformation of the exponential form (e^(I KX - Omega T)) into a combination of cosine and sine functions, representing the wave's amplitude and phase.
What is the role of the potential energy (V) in the context of the wave equation?
-In the context of the wave equation, the potential energy (V) is a term that represents the energy of the system due to its position in a force field. When V is constant, it simplifies the Schrödinger equation, allowing the wave equation to be used for calculating probabilities.
How does the total energy of a particle in the wave equation relate to its kinetic and potential energy?
-In the wave equation, the total energy of a particle is the sum of its kinetic and potential energy. This is reflected in the Schrödinger equation, where the kinetic energy term and the potential energy term together determine the form of the wave equation.
Outlines
🌊 Introduction to Wave Equation in Quantum Mechanics
This paragraph introduces the concept of the wave equation in the context of quantum mechanics. It begins by discussing the physical meaning of the wave equation and its representation of a particle subjected to various physical constraints such as forces, potential energy, and kinetic energy. The paragraph then connects the wave equation to the Schrödinger equation, highlighting the role of the wave equation in describing the probability of finding a particle at a particular location rather than representing a physical wave or a measurable classical wave function. The importance of understanding the wave equation's role in quantum mechanics is emphasized, as it provides a means to calculate probabilities in a quantum system.
Mindmap
Keywords
💡Wave Equation
💡Schrodinger Equation
💡Differential Equation
💡Particle
💡Potential Energy
💡Kinetic Energy
💡Real Solution
💡Probability
💡Complex Numbers
💡Quantum Mechanics
Highlights
Exploring the physical meaning of the wave equation.
The wave equation's representation of a particle under physical constraints.
The connection between the wave equation and the Schrödinger equation.
The role of the wave equation in satisfying the Schrödinger equation.
The wave equation's non-real nature and its implications for quantum mechanics.
The use of complex numbers (I) in the wave equation.
The reduction of the Schrödinger equation to a constant potential scenario.
The transition from the Schrödinger equation to a classical mechanics equation.
The wave equation's ability to calculate the probability of finding a particle at a location.
The distinction between the wave equation and a measurable classical wave function.
The importance of the wave equation in quantum mechanics for probability calculation.
The non-intuitive aspect of the wave equation in quantum mechanics.
The upcoming explanation of the technique to calculate probability using the wave equation.
The fundamental role of the wave equation in understanding quantum mechanics.
The challenge of imagining a wave equation that doesn't represent a real wave in quantum mechanics.
Transcripts
Browse More Related Video
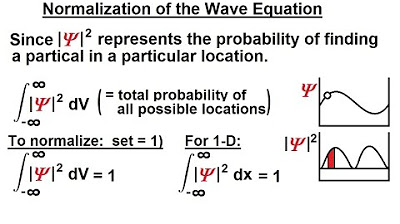
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (2 of 92) Normalization of the Wave Equation

The Quantum Wavefunction Explained
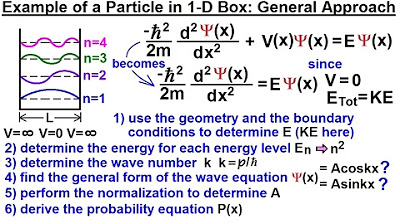
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (18 of 92) Particle in 1-D Box: Gen. Appr.
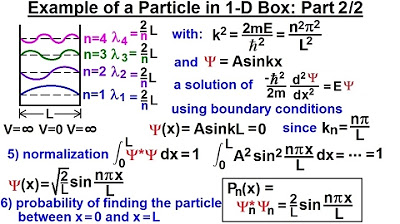
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (20 of 92) Particle in 1-D Box: Example 2/2
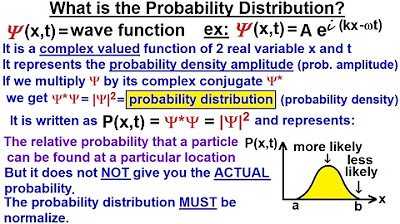
Physics - Ch 66 Ch 4 Quantum Mechanics: Schrodinger Eqn (9 of 92) What is the Prob Distribution?
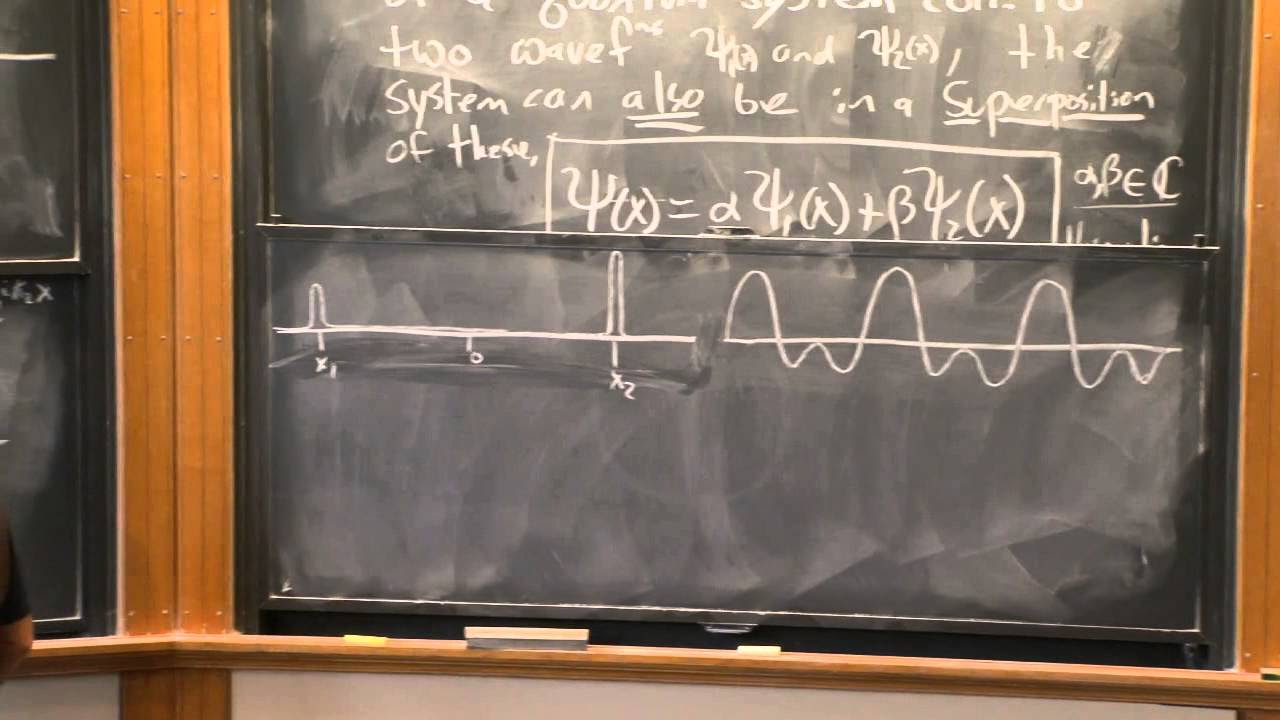
Lecture 3: The Wave Function
5.0 / 5 (0 votes)
Thanks for rating: