Understanding the Atmosphere | Essentials of Environmental Science
TLDRThe script delves into the importance of Earth's atmosphere, a thin layer that sustains life. It explores how human activities, particularly fossil fuel burning from vehicles and power plants, have negatively impacted air quality by releasing pollutants like nitrogen and sulfur oxides, particulate matter, carbon monoxide, and ground-level ozone. It highlights the detrimental effects of these air pollutants on human health, ecosystems, and climate. The script also discusses the progress made through regulations like the Clean Air Act and the Montreal Protocol in reducing air pollution and ozone depletion, while emphasizing the need for continued efforts to achieve cleaner air.
Takeaways
- 🌎 The atmosphere is a thin layer of gases surrounding the Earth, with a composition primarily of nitrogen, oxygen, and small amounts of other gases like carbon dioxide.
- ☢️ The ozone layer in the stratosphere protects life on Earth by absorbing harmful ultraviolet radiation from the Sun.
- 🏭 Human activities, particularly burning fossil fuels in vehicles and power plants, release pollutants like nitrogen oxides, sulfur oxides, particulate matter, lead, carbon monoxide, and ground-level ozone into the atmosphere.
- ☠️ Air pollution is a major environmental concern, causing an estimated 5-6 million premature deaths annually due to respiratory issues and other health problems.
- 🚗 Reducing emissions from fossil fuel combustion, such as through regulations, technological improvements, and a transition to cleaner energy sources, can help mitigate air pollution.
- 🌱 The Clean Air Act in the United States has led to significant reductions in air pollutant levels since its implementation, demonstrating the effectiveness of environmental legislation.
- 🔬 The story of stratospheric ozone depletion and the Montreal Protocol highlights how scientific understanding, identification of the problem, and international cooperation can address environmental issues.
- 🏡 Indoor air pollution from sources like gas stoves and household products poses additional health risks and is more challenging to regulate.
- 🌡️ Carbon dioxide, while not directly harmful to human health like other air pollutants, is a significant greenhouse gas contributing to climate change.
- 🌍 Addressing air pollution requires a multifaceted approach, involving regulations, technological advancements, and a global commitment to reducing fossil fuel consumption and promoting sustainable practices.
Q & A
What is the significance of the earth's atmosphere?
-The earth's atmosphere, although relatively thin, is crucial for sustaining life on the planet. It acts as a protective layer, shielding organisms from harmful ultraviolet radiation and providing the necessary gases for respiration and other biological processes.
What is the primary concern regarding the ozone layer?
-The ozone layer in the stratosphere is vital as it absorbs a significant portion of the sun's harmful ultraviolet radiation. Depletion of the ozone layer due to the release of substances like chlorofluorocarbons (CFCs) can expose life on earth to increased levels of UV radiation, leading to various health issues.
What are the main sources of air pollution discussed in the script?
-The script primarily highlights vehicles, power plants (particularly those that burn fossil fuels), and certain industrial processes as the main sources of air pollution.
What are the six criteria pollutants monitored by the Environmental Protection Agency (EPA)?
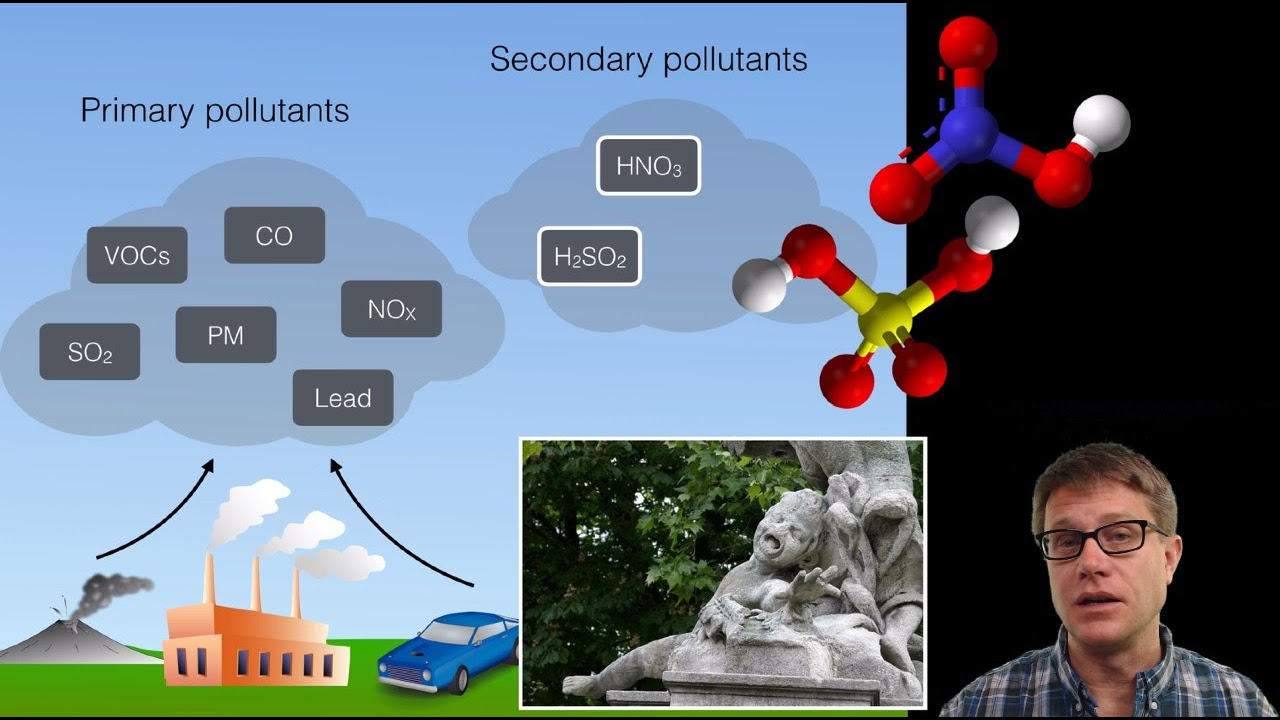
-The six criteria pollutants monitored by the EPA are nitrogen oxides (NOx), sulfur oxides (SOx), particulate matter, lead, carbon monoxide, and ozone.
What is the significance of particulate matter as an air pollutant?
-Particulate matter, which includes solid particles like smoke, dust, and soot, can be harmful to human health, particularly when the particles are smaller (PM2.5). Some particulate matter can also contribute to the formation of photochemical smog and acid rain.
How does the Clean Air Act help address air pollution in the United States?
-The Clean Air Act gives the Environmental Protection Agency (EPA) the authority to set limits on air pollutants and enforce those limits. It has led to significant decreases in various air pollutants, such as carbon monoxide, sulfur dioxide, volatile organic compounds (VOCs), nitrogen oxides (NOx), and particulate matter, since its implementation.
What is the difference between stratospheric ozone and tropospheric ozone?
-Stratospheric ozone is beneficial as it forms the ozone layer that protects the earth from harmful UV radiation. On the other hand, tropospheric or ground-level ozone is a human-made secondary air pollutant that can be harmful to human health and contribute to the formation of photochemical smog.
What is the role of carbon dioxide in the context of air pollution and climate change?
-While carbon dioxide is not typically considered an air pollutant harmful to human health, it is a significant greenhouse gas that plays a crucial role in climate change. The script mentions that carbon dioxide, along with methane and nitrous oxide, contributes to the warming of the planet when present in high concentrations.
What is the significance of the Montreal Protocol in addressing air pollution?
-The Montreal Protocol is cited as an example of international cooperation in addressing the issue of ozone depletion. It led to the banning of chlorofluorocarbons (CFCs), which were responsible for depleting the ozone layer, and has contributed to the recovery of the earth's ozone layer.
What are some potential solutions mentioned in the script to reduce air pollution?
-The script suggests reducing the burning of fossil fuels, transitioning away from coal-fired power plants, and reducing emissions from internal combustion engines as potential solutions to mitigate air pollution.
Outlines
🌫️ The Atmosphere and Its Layers
This paragraph introduces the atmosphere as a thin layer surrounding the Earth that is essential for sustaining life. It describes the composition of the atmosphere, its various layers (troposphere, stratosphere, mesosphere, thermosphere, and exosphere), and the significance of the troposphere and stratosphere. It highlights the ozone layer in the stratosphere, its role in blocking harmful UV radiation, and the consequences of its depletion caused by chlorofluorocarbons (CFCs). The paragraph emphasizes the importance of the ozone layer for life on Earth and the successful international efforts to ban CFCs and mitigate ozone depletion.
🏭 Air Pollutants and Their Sources
This paragraph delves into the different types of air pollutants and their sources. It introduces the six criteria pollutants monitored by the Environmental Protection Agency (EPA): nitrogen oxides (NOx), sulfur oxides (SOx), particulate matter, lead, carbon monoxide, and ground-level ozone. The paragraph explains the formation and harmful effects of these pollutants, with a focus on emissions from power plants and vehicles burning fossil fuels. It discusses the impacts of acid deposition, particulate matter (including natural and anthropogenic sources), lead, carbon monoxide, and ground-level ozone formation (photochemical smog). The paragraph emphasizes the adverse effects of these pollutants on human health, ecosystems, and the environment.
💨 Addressing Air Pollution and Climate Change
This paragraph explores the efforts to address air pollution and its impacts. It highlights the improvements in air quality in the United States through regulations like the Clean Air Act, which empowered the Environmental Protection Agency (EPA) to set and enforce limits on air pollutants. The paragraph discusses the decrease in emissions of various pollutants since the Clean Air Act's implementation. It also mentions indoor air pollutants and their challenges in regulation. Additionally, the paragraph introduces the role of carbon dioxide as a greenhouse gas contributing to climate change, distinguishing it from traditional air pollutants. The paragraph concludes by emphasizing the need for continued efforts, including strengthening regulations and reducing fossil fuel use, to address air pollution and its impacts on human health, ecosystems, and the environment.
Mindmap
Keywords
💡Atmosphere
💡Ozone Layer
💡Air Pollution
💡Criteria Pollutants
💡Fossil Fuels
💡Photochemical Smog
💡Acid Rain
💡Particulate Matter
💡Clean Air Act
💡Carbon Dioxide (CO2)
Highlights
The atmosphere is extremely thin, like a single layer of plastic wrap around a basketball, yet it sustains life on Earth.
Burning fossil fuels, especially from vehicles and power plants, is a major source of air pollutants.
The troposphere is where most environmental science phenomena occur, including all life forms, biogeochemical cycles, and weather patterns.
The stratospheric ozone layer blocks out harmful UV radiation, making life on Earth possible as we know it.
The ozone hole was caused by chlorofluorocarbons (CFCs), which led to international cooperation and a ban under the Montreal Protocol in 1987.
Air pollution is a broad category that encompasses anything in the air that harms organisms, and it causes an estimated 5-6 million deaths annually.
The EPA monitors six criteria pollutants: NOx, SOx, particulate matter, lead, carbon monoxide, and ozone.
NOx and SOx are highly reactive and can cause acid rain, damaging buildings, plants, and organisms.
Particulate matter, especially from power plants, can contain harmful substances like mercury and lead, posing health risks.
Lead is a neurotoxin that affects cognition and brain development, and it persists in the environment for a long time.
Carbon monoxide is a colorless, odorless, and highly toxic gas that prevents oxygen from being carried in the blood.
Ground-level ozone, a secondary air pollutant, is formed by the reaction of NOx, volatile organic compounds (VOCs), and sunlight, contributing to photochemical smog.
The Clean Air Act gave the EPA authority to set and enforce limits on air pollutants, leading to significant improvements in air quality in the United States.
Indoor air pollutants, which occur inside homes, are harder to test for and regulate.
While carbon dioxide is not directly harmful to human health, it plays a crucial role as a greenhouse gas contributing to climate change.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: