ALEKS: Identifying oxidized and reduced reactants in a single-displacement reaction
TLDRThis educational video script guides viewers on identifying oxidized and reduced reactants in single displacement reactions. It defines oxidation as the loss of electrons and reduction as the gain, using examples to illustrate these processes. The script then demonstrates how to analyze balanced equations to pinpoint the reactant undergoing oxidation by looking for an atom turning into a cation and the one undergoing reduction by identifying a cation turning into an atom. It emphasizes the importance of recognizing the sequence in ionic compounds and concludes with a practice exercise, highlighting the exclusion of stoichiometric coefficients and states in the final answer.
Takeaways
- 🔬 Oxidation is the loss of electrons, exemplified by a neutral atom becoming a positively charged cation.
- 🌗 Reduction is the gain of electrons, where a cation gains electrons and is converted back to an atom.
- 📚 The examples given are specific to the Alex problem and may not apply to all homework problems.
- 🧩 In a single displacement reaction, one reactant is oxidized while the other is reduced.
- 👉 To identify the oxidized reactant, look for an atom that is converted into a cation in the reaction.
- 🔄 The reactant being reduced is the one that is a cation and is converted into an atom.
- 📝 In the balanced equation, the first substance listed in an ionic compound is the cation, and the second is the anion.
- 📚 The process of identifying oxidized and reduced reactants involves recognizing the change from atom to cation for oxidation and cation to atom for reduction.
- 📉 When writing the reduced reactant, include the entire ionic compound formula, not just the element.
- ⚠️ Do not include stoichiometric coefficients when identifying the reactants in the Alex problem.
- 📑 The Alex problem does not require including states of matter in the answers.
Q & A
What is oxidation in the context of the video?
-Oxidation is defined as the loss of electrons, such as a neutral atom losing electrons and becoming a positively charged cation.
What is reduction in the context of the video?
-Reduction is the gain of electrons, which is the opposite of oxidation, like a cation gaining electrons and being converted back into an atom.
Why is it important to distinguish between oxidation and reduction in a chemical reaction?
-It is important because in an oxidation-reduction (redox) reaction, one reactant is oxidized while the other is reduced, and understanding this helps in identifying the changes in the reactants.
How does the video define the oxidized reactant in a single displacement reaction?
-The oxidized reactant is identified as an atom that is converted into a cation in the reaction.
How does the video define the reduced reactant in a single displacement reaction?
-The reduced reactant is identified as a cation that is converted back into an atom.
What is the significance of the order of substances in an ionic compound formula?
-In an ionic compound formula, the first substance listed is the cation and the second is the anion, which helps in identifying the charge of the ions.
Can both reactants in a redox reaction be oxidized or reduced at the same time?
-No, in a redox reaction, one reactant is oxidized while the other is reduced; both cannot be oxidized or reduced simultaneously.
How does the video suggest identifying the reduced reactant in a redox problem?
-The video suggests that since one reactant is oxidized, the other is automatically reduced, so identifying one helps in identifying the other.
What is the role of stoichiometric coefficients in the context of the video?
-The video mentions that stoichiometric coefficients are not necessary to include when identifying oxidized and reduced reactants in the context of the Alex problem.
Why is it incorrect to include the stoichiometric coefficients when writing the ionic formula of the reduced reactant?
-Including the stoichiometric coefficients would be incorrect because the Alex problem does not require states or coefficients for the answer.
Does the Alex problem require including the states of the reactants in the answer?
-No, the Alex problem does not require including the states of the reactants in the answer.
Outlines
🔬 Understanding Oxidation and Reduction in Displacement Reactions
This paragraph introduces the concepts of oxidation and reduction in the context of single displacement reactions. Oxidation is defined as the loss of electrons, exemplified by a neutral atom becoming a positively charged cation. Conversely, reduction is the gain of electrons, where a cation becomes an atom. The paragraph emphasizes that while these examples are specific to the problem at hand, there are various types of oxidation and reduction that may apply to different scenarios. The speaker also explains the process of identifying oxidized and reduced reactants by looking for atoms that transform into cations (oxidation) or cations that revert to atoms (reduction).
Mindmap
Keywords
💡Oxidation
💡Reduction
💡Single Displacement Reaction
💡Cation
💡Anion
💡Ionic Compound
💡Stoichiometric Coefficient
💡States of Matter
💡Balanced Equation
💡Reactant
💡Oxidation State
Highlights
Introduction to solving the Alex problem of identifying oxidized and reduced reactants in a single displacement reaction.
Definition of oxidation as the loss of electrons, with an example of an atom becoming a positively charged cation.
Definition of reduction as the gain of electrons, with an example of a cation converting back into an atom.
Clarification that the examples provided are specific to the Alex problem and may not apply to all homework problems.
Explanation of how to identify an oxidized reactant by looking for an atom being converted into a cation.
Method to identify the oxidized reactant in a balanced equation by finding the atom that becomes a cation.
Understanding that the first substance listed in an ionic compound is the cation, helping to identify the oxidized reactant.
Identification of the reduced reactant as the one that is always the opposite of the oxidized reactant in any redox problem.
Guidance on identifying the reduced reactant by looking for a cation being converted into an atom.
Emphasis on writing the whole ionic compound when identifying the reduced reactant, not just the element.
Practice with a second balanced equation to reinforce the method of identifying oxidation and reduction.
Illustration of identifying oxidation by finding an atom that gets converted into a cation in a balanced equation.
Instruction on writing the whole ionic formula for the reduced reactant, excluding the stoichiometric coefficient.
Note that the Alex problem does not require including states in the answer.
Highlight of the importance of understanding the definitions of oxidation and reduction to solve the Alex problem.
Reinforcement that identifying one reactant in a redox reaction helps automatically identify the other due to their complementary nature.
Final note on the necessity of leaving out stoichiometric coefficients when writing the ionic formula for the reduced reactant.
Transcripts
Browse More Related Video

How to Balance Redox Equations in Acidic Solution

Oxidation and Reduction (Redox) Reactions Step-by-Step Example

GCSE Chemistry - Oxidation and Reduction - Redox Reactions #39 (Higher Tier)
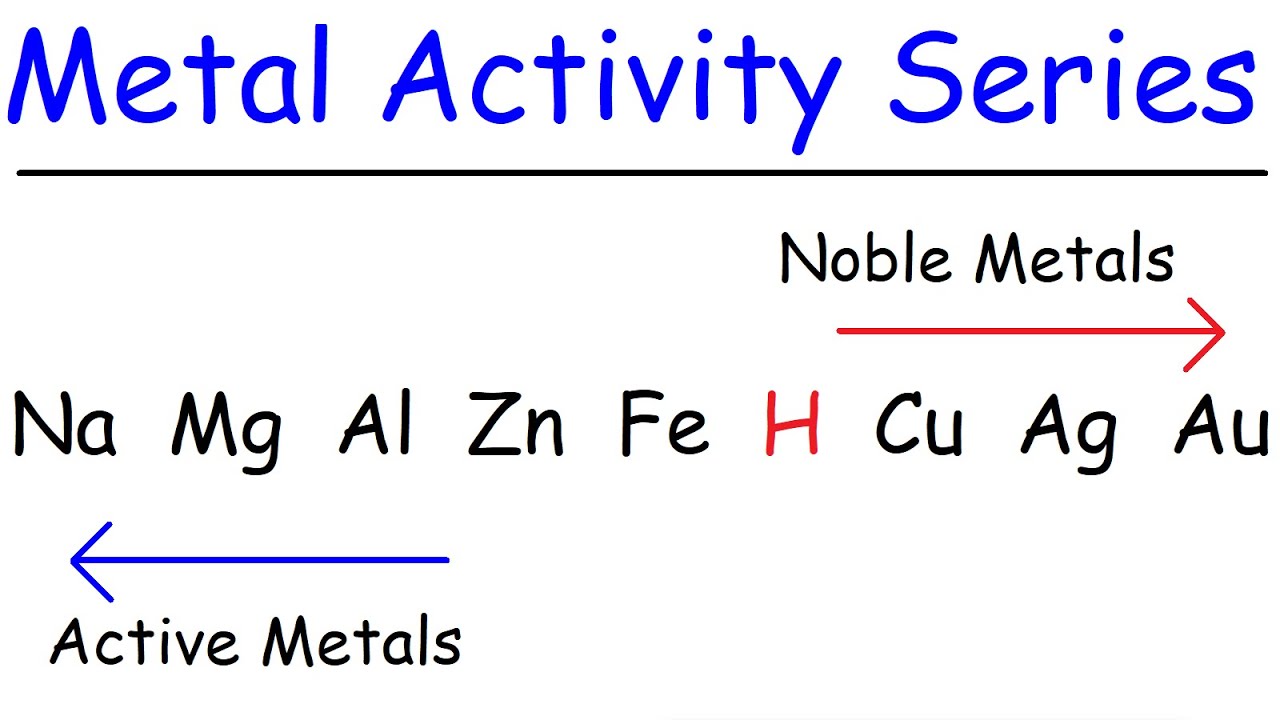
Activity Series of Metals - Chemistry

Redox Reactions
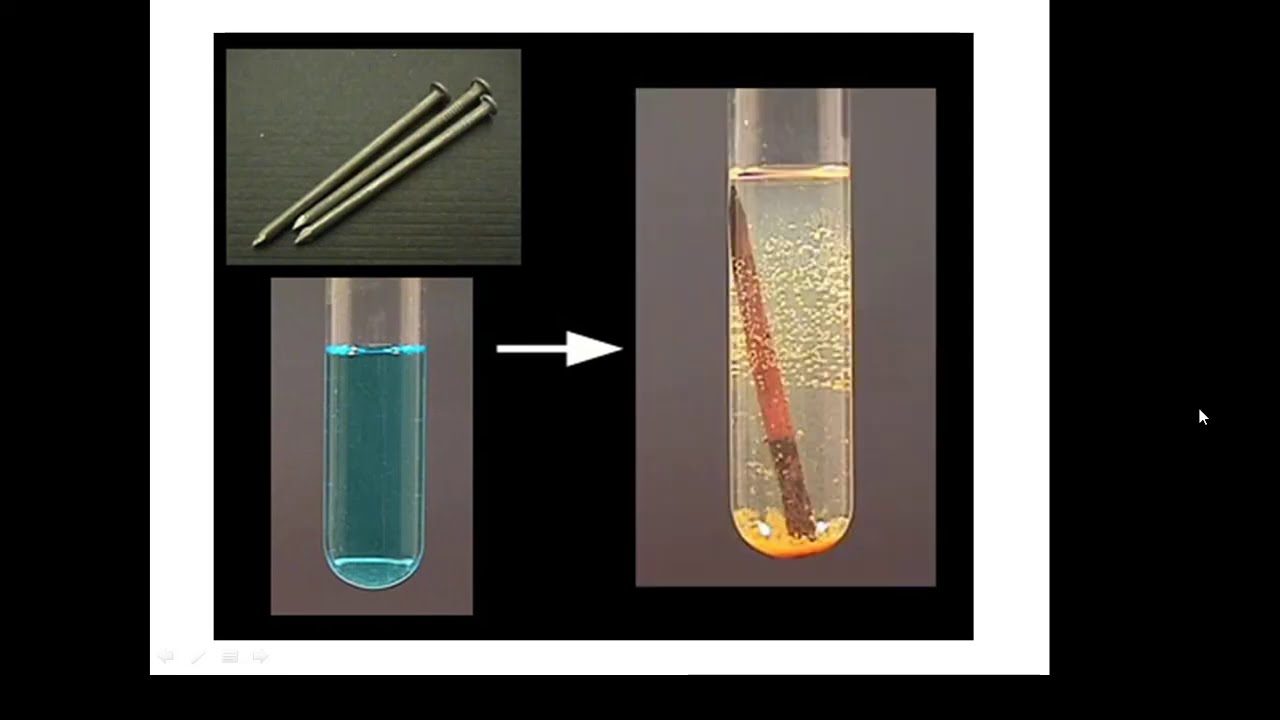
BTEC Applied Science: Unit 1 Chemistry Displacement Reactions
5.0 / 5 (0 votes)
Thanks for rating: