BTEC Applied Science: Unit 1 Chemistry Displacement Reactions
TLDRThis educational video script delves into the fundamentals of displacement reactions, oxidation, and reduction in chemistry. It explains the transformation of iron and copper sulfate, highlighting the reactivity series and how it dictates whether a reaction will occur. The concept of halogens' reactivity is introduced, and the script emphasizes the importance of understanding oxidation as the loss of electrons and reduction as the gain, both integral to redox reactions. The benefits of using carbon in industrial processes, such as the blast furnace, are also discussed, providing a practical application of these chemical principles.
Takeaways
- 🌟 Displacement reactions involve a more reactive metal displacing a less reactive metal from its compound.
- 🔩 When iron is placed in copper sulfate, it turns orange due to the deposition of copper, and the solution changes from blue to pale orange as it becomes iron sulfate.
- 🏆 The reactivity series is crucial in determining whether a displacement reaction will occur or not.
- 🎯 Magnesium and iron can displace copper, but zinc cannot displace calcium due to their relative reactivities.
- 🌊 Halogens (group 7 elements) can also participate in displacement reactions, with more reactive halogens displacing less reactive ones.
- 🔋 Oxidation is the process of a metal atom losing electrons, while reduction is the gain of electrons by a metal ion.
- 📈 Half equations are used to represent the electron transfer in redox reactions, showing the oxidation and reduction processes for individual atoms and ions.
- 🛠️ In the reaction between iron and copper sulfate, iron atoms are oxidized, and copper ions are reduced.
- 📝 Practice is essential for understanding and applying the concepts of displacement reactions, oxidation, and reduction.
- 🔥 In a blast furnace, carbon is used to displace iron from iron oxide due to its reactivity and advantages over other metals like aluminum, magnesium, or calcium.
Q & A
What is the main topic of discussion in the transcript?
-The main topic of discussion in the transcript is displacement reactions, particularly in the context of chemistry involving metals and their reactivity, as well as the concepts of oxidation and reduction.
What happens when an iron nail is placed in copper sulfate solution?
-When an iron nail is placed in copper sulfate solution, a displacement reaction occurs. The iron nail turns orange as it gets covered in copper, and the copper sulfate solution changes to a pale orange color as it becomes iron sulfate. This happens because iron is more reactive than copper and displaces it from the solution.
What is a displacement reaction?
-A displacement reaction is a type of chemical reaction where one element replaces another in a compound. This typically occurs when a more reactive metal displaces a less reactive metal from its compound, resulting in the formation of a new element and a new compound.
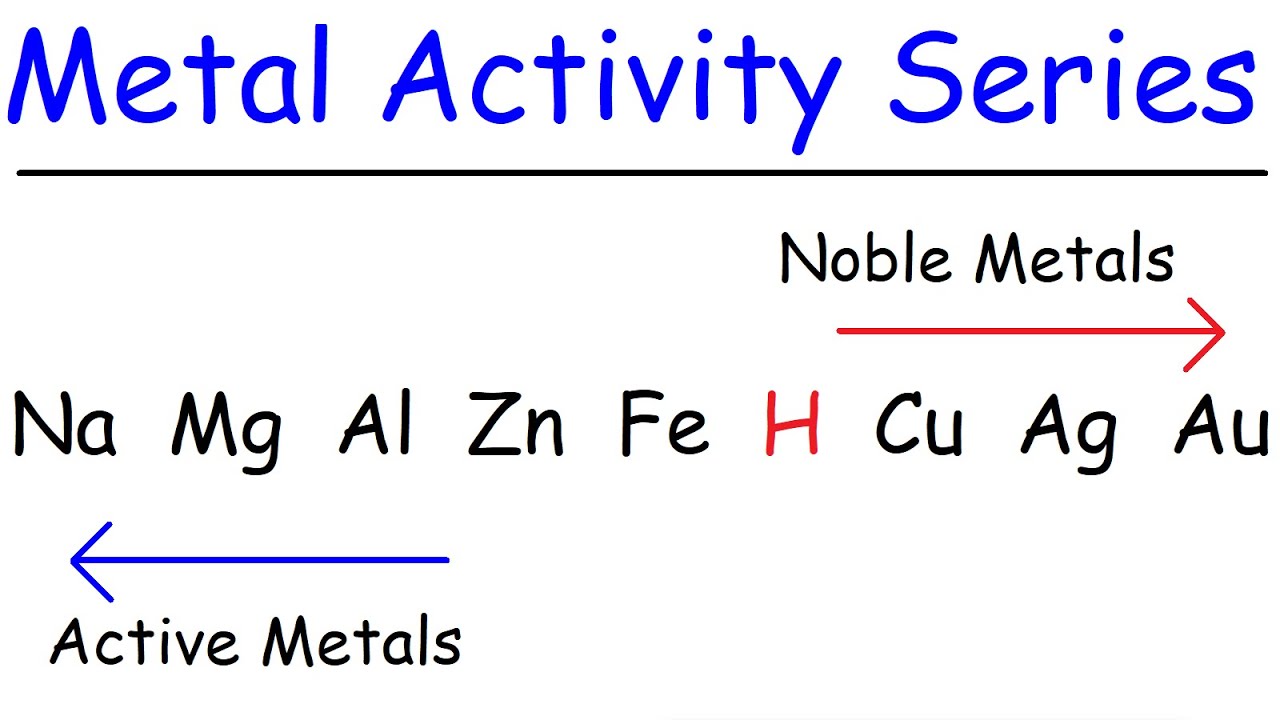
What is the reactivity series mentioned in the transcript, and why is it important?
-The reactivity series is a list of metals arranged in order of decreasing reactivity. It is important because it helps predict the outcomes of displacement reactions. Only a more reactive metal can displace a less reactive one from its compound.
How can halogens be involved in displacement reactions?
-Halogens can be involved in displacement reactions where a more reactive halogen can displace a less reactive one. For example, chlorine can displace bromine from sodium bromide to form sodium chloride and bromine.
What is oxidation in the context of the script?
-In the context of the script, oxidation refers to the loss of one or more electrons by a metal atom, resulting in the formation of a positively charged ion.
What is reduction in the context of the script?
-Reduction, in the context of the script, is the gain of one or more electrons by a metal ion, resulting in the formation of an element in its neutral atomic form.
What is a half equation?
-A half equation is a chemical equation that shows the change in terms of electrons for either the oxidation or reduction process separately. It helps to understand the electron transfer involved in redox reactions.
What is a redox reaction?
-A redox reaction is a chemical reaction that involves both oxidation and reduction occurring simultaneously. It is characterized by the transfer of electrons from one species to another.
Why is carbon used in a blast furnace instead of other reactive metals like aluminum or magnesium?
-Carbon is used in a blast furnace to displace iron from iron oxide due to its reactivity and cost-effectiveness. While other metals like aluminum or magnesium are more reactive, they are more expensive or less readily available, making carbon a more practical choice.
What can be inferred about hydrogen and carbon from their placement on the reactivity series?
-From their placement on the reactivity series, it can be inferred that both hydrogen and carbon can exhibit metallic properties under certain conditions. Hydrogen, due to its position on the periodic table, and carbon, due to its ability to act like a metal in certain industrial processes such as the production of iron in a blast furnace.
Outlines
🔬 Introduction to Displacement Reactions and Reactivity Series
This paragraph introduces the concept of displacement reactions, using the example of an iron nail being placed in copper sulfate solution. It explains the observable changes, the underlying chemical reaction, and the principle of displacement reactions where a more reactive metal displaces a less reactive one. The paragraph also touches on the reactivity series, highlighting that reactions occur only if the metal at the start is more reactive. The introduction of halogens and their role in displacement reactions is also discussed, emphasizing the unique reactivity order among them. The paragraph sets the stage for understanding redox reactions and the reactivity series' significance in predicting reaction outcomes.
📚 Understanding Oxidation, Reduction, and Half Equations in Displacement Reactions
The second paragraph delves deeper into the concepts of oxidation and reduction within the context of displacement reactions. It explains how one metal is oxidized while the other is reduced, using the iron and copper sulfate example to illustrate these processes. The paragraph introduces half equations as a method to represent the electron transfer involved in these reactions, providing clarity on the atomic and ionic changes. It also poses questions to engage the audience, encouraging them to apply their understanding of reactivity series and predict reaction outcomes. Additionally, the paragraph discusses the unique role of hydrogen and carbon in the reactivity series, mentioning their metallic-like behavior in certain industrial processes such as the extraction of iron in a blast furnace. The advantages of using carbon over other reactive metals are also hinted at, setting the stage for further exploration into the practical applications of these concepts.
Mindmap
Keywords
💡Displacement Reaction
💡Oxidation
💡Reduction
💡Reactivity Series
💡Halogens
💡Iron Sulfate
💡Copper Sulfate
💡Half Equations
💡Redox Reactions
💡Blast Furnace
💡Carbon
Highlights
Introduction to displacement reactions with an example of iron nail in copper sulfate.
Observation that the iron nail turns orange and copper sulfate changes color due to a chemical reaction.
Chemical equation for the reaction between iron and copper sulfate resulting in copper and iron sulfate.
Explanation of the displacement reaction where iron takes the place of copper, demonstrating the reactivity series.
Mention of the reactivity series and its importance in determining the occurrence of displacement reactions.
Example of magnesium reacting with copper sulfate due to its higher reactivity.
Example of no reaction occurring between zinc and calcium sulfate due to zinc being less reactive than calcium.
Discussion on halogens and their displacement reactions, with more reactive halogens displacing less reactive ones.
Explanation of oxidation and reduction in the context of metal atoms and ions, defining oxidation as loss of electrons and reduction as gain of electrons.
Introduction to half equations to represent the electron transfer in redox reactions.
Description of a redox reaction where one metal is oxidized and the other is reduced, using the example of iron and copper sulfate.
Writing of half equations for copper ions gaining electrons and iron atoms losing electrons.
A video recommendation for further understanding of redox reactions.
Question about predicting the outcome of a reaction between magnesium and copper chloride, including writing word and half equations.
Discussion on hydrogen and carbon's position on the reactivity series and their occasional metallic-like behavior.
Explanation of carbon's use in a blast furnace to displace iron from iron oxide and the advantages over other elements like aluminum or magnesium.
Transcripts
Browse More Related Video

Oxidation and Reduction Reactions - Basic Introduction
Activity Series of Metals - Chemistry

Oxidation vs. Reduction, What are Oxidation and Reduction Reactions in Everyday Life?

Redox Reactions: Crash Course Chemistry #10

Redox Reactions

What are Oxidation and Reduction Reactions in Everyday Life? (Revised with better sound quality)
5.0 / 5 (0 votes)
Thanks for rating: